Composition and application of novel medicine package for preventing and treating women's cervical virus infection
A virus infection and drug package technology, applied in the field of novel drug delivery package, can solve the problems that the drug cannot directly reach the lesion, the drug has a short action time, and the dosage is insufficient, so as to prolong the staying time, promote the repair, and increase the dosage of the drug. accurate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Embodiment 1: The drug delivery package designed according to the anatomical characteristics of female vagina
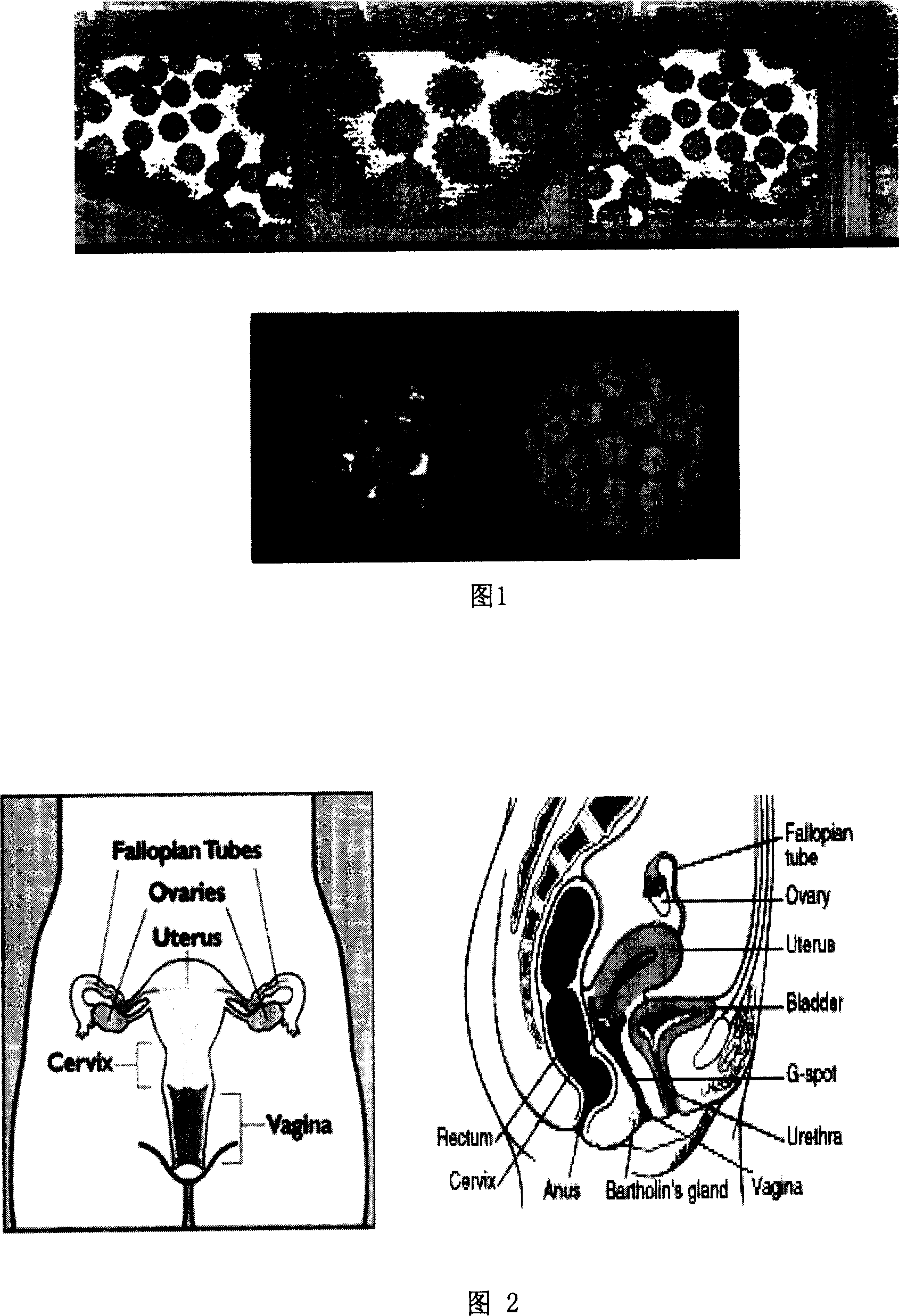
[0120] Female internal genitalia is composed of vagina, cervix, uterus, fallopian tubes and ovaries, as shown in Figure 2 of the description.
[0121] Vagina: The vagina is cylindrical, a highly expansive, muscular lumen, divided into anterior and posterior walls, with upper and lower ends; the anterior wall is shorter, about 7-9 cm; the rear wall is longer, about 10-12 cm . The upper end is wider and surrounds the cervix. The part surrounding the cervix is called the vaginal vault, which is divided into four parts: front, back, left, and right. The cervix and vagina protrude from the upper end of the vagina, and the lower end opens at the back of the vaginal vestibule, which is narrower than the upper end. Usually, the front and back walls of the vagina are close together, making the cross section of the lower part of the vagina H-shaped. From the vestib...
Embodiment 2
[0125] Embodiment 2: The vaginal administration package we invented contains 1.0ml of the labeled amount of the prescription drug, and the prescription is as follows:
[0126] Recombinant human interferon α2b (Escherichia coli): 3 million IU / ml
[0127] Human Albumin: 0.5%
[0129] Chitosan: 0.1%
[0130] Acetate buffer (pH4.5): 50mM
[0131] Dissolve chitosan, prepare 1% mother solution, and prepare 1M acetate buffer solution (pH4.5), then mix the components according to the prescription concentration, sterilize and filter, and pack until sterilized In the above-mentioned bag containing the drug pack, seal it.
Embodiment 3
[0132] Embodiment 3: Each vaginal administration package contains 1.0ml of the labeled amount of the prescription drug, and the prescription can be as follows:
[0133] Recombinant human interferon beta: 3 million IU / ml
[0134] Human Albumin: 0.5%
[0135] Recombinant Human Interleukin-2 200,000 IU / ml
[0137] Hydroxypropyl methylcellulose: 0.5%
[0138] Citrate buffer (pH5.0): 50mM
[0139] Dissolve hydroxypropyl methylcellulose, prepare 2% mother solution, and prepare 1M citrate buffer solution (pH5.0), then mix the components according to the prescription concentration, sterilize and filter, and pack Put it into the sterilized bag containing the above-mentioned drug pack, and seal it. Embodiment 4: Each vaginal administration package contains 1.0ml of the labeled amount of the prescription drug, and the prescription can be as follows:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com