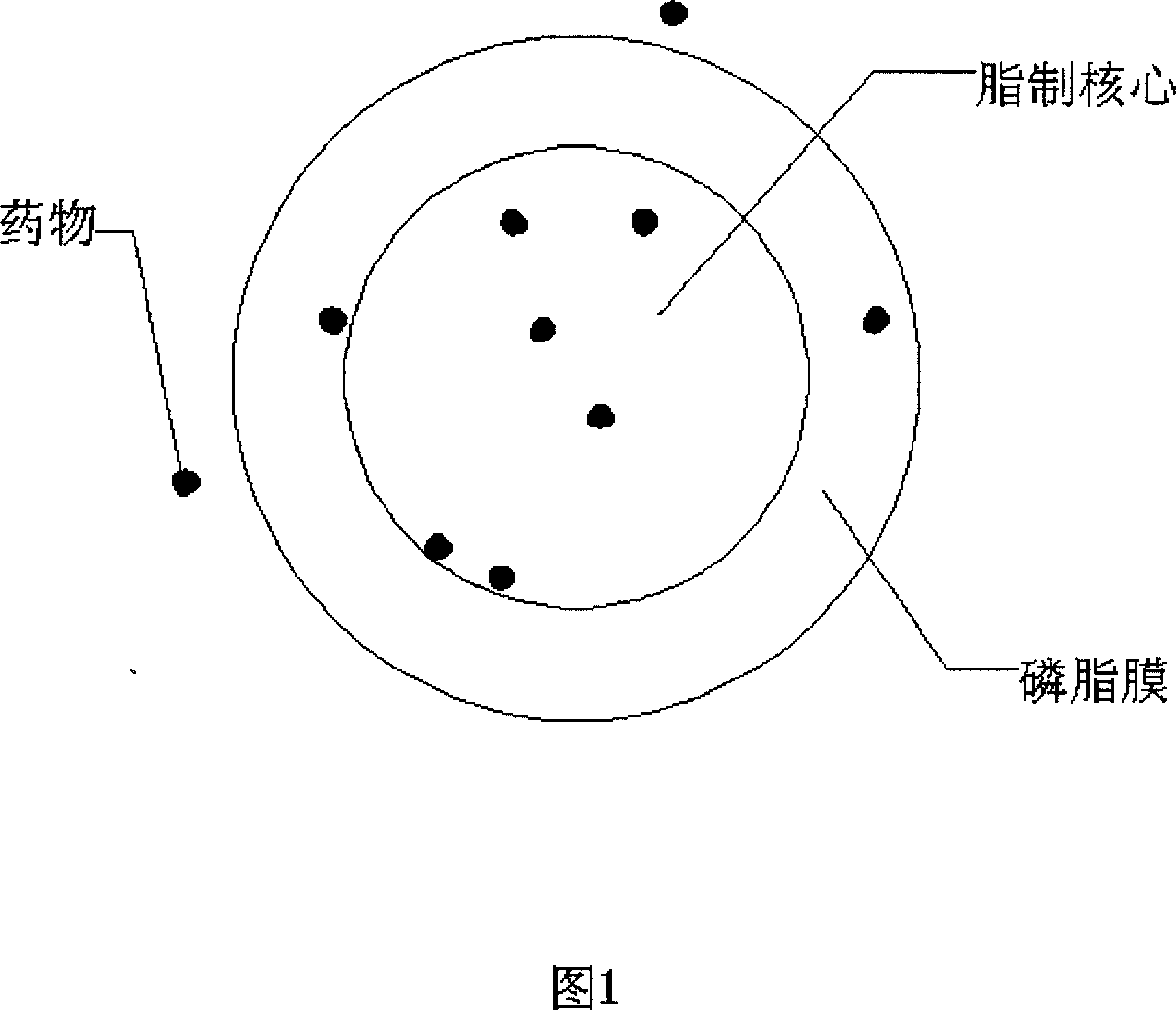
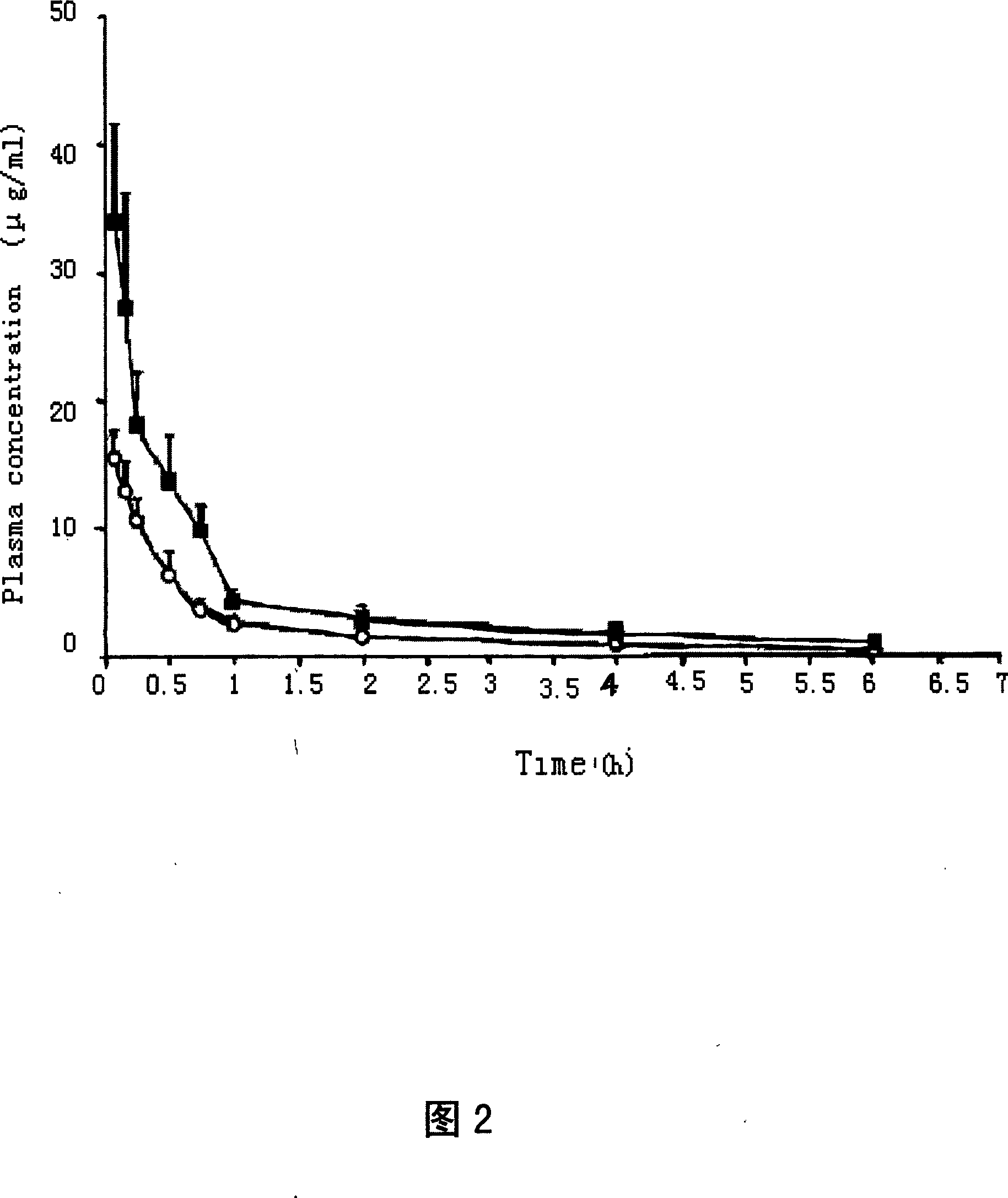
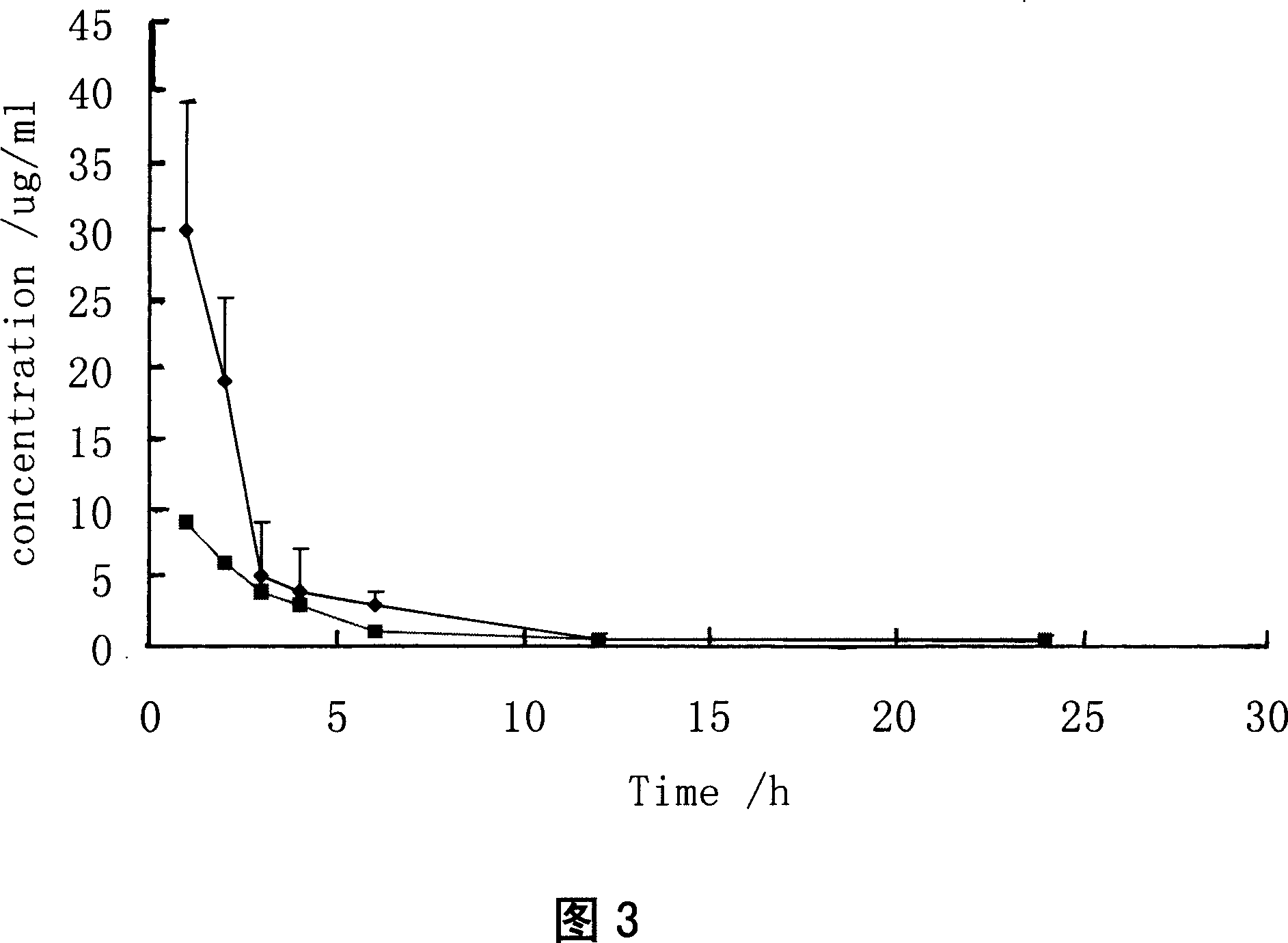
Injection containing lipoid microsphere of etoposide and its prepn process
A technology of etoposide and lipid microspheres, which is applied in the field of medicine, can solve problems such as poor water solubility, interference with drug effects, and toxicity, and achieve the effects of reducing toxic side effects, reducing vascular irritation, and strong innovation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The prescription preparation technology of embodiment 1 etoposide preparation
[0048] Prescription 1:
[0049] Safflower oil for injection 20g
[0050] Etoposide 0.2g
[0051] Lecithin 1.8g
[0052] Glycerin 2.5g
[0053] Add water for injection to 100ml
[0054] Preparation method 1:
[0055] (1) Mix the prescribed amount of glycerin with an appropriate amount of water for injection preheated to 40-100°C, transfer it to a tissue grinder, and stir for several minutes, 1-5 times, until the ingredients are dissolved to obtain an aqueous phase; at the same time Add lecithin to the prescribed amount of safflower oil and heat to dissolve at 40-100°C to obtain the oil phase; (2) Add the oil phase to the water phase, transfer it to a tissue grinder, stir for several minutes, 1-5 times, Until the oil phase is uniformly dispersed to obtain colostrum, adjust the pH value to 4-8; (3) Add etoposide drug powder to the colostrum, stir for 10 minutes, add water for injection pr...
Embodiment 2
[0096] Example 2 The investigation of the stability of etoposide lipid microspheres
[0097] According to the experimental method reported in the literature: the etoposide lipid microsphere sample was washed with redistilled water, buffer salt (Na 2 HPO 4 , 0.017M; KH 2 PO 4 , 0.0014M; NaCl, 0.1370M, pH7.4) and glycerol (2.21% w / w) were diluted, and the particle size, ζ-potential and drug encapsulation rate after dilution were measured 1h, 2h, 4h, 24h, and 48h. The etoposide lipid microspheres were stored at 4-8°C and 25°C for 6 months, and the particle size and encapsulation rate of the samples were measured at different time points. The test results showed that the particle size of the etoposide lipid microspheres was not affected by weight. Effect of distilled water, buffer saline (pH 7.4) and glycerol (2.21% w / w) dilution. After 48 h, the particle size in redistilled water and glycerol hardly changed, while in buffer salt (pH 7.4), the particle size increased by 50 nm....
Embodiment 3
[0098] Example 3 Irritation Test of Etoposide Lipid Microsphere Injection
[0099] (1) Vascular stimulation test
[0100] Two different dosage forms of etoposide injection were converted according to the clinical dosage (100mg / time) to obtain the experimental rabbit dose (5.2mg / kg) by body surface area conversion. Before the test, it was freshly prepared with sterile physiological saline injection according to the dosage of 2ml / kg. Six healthy New Zealand white rabbits with a body weight of 2.5-3.0 kg were selected, both male and female. After the injection site was disinfected with tincture of iodine and ethanol, 3 white rabbits were injected with etoposide water injection (Cs) in the ear vein of the right ear, and the left ear was injected with the same dose of sterile normal saline injection as a control; Rabbits were injected with etoposide lipid microsphere injection (Cz) in the ear vein of the right ear, and the same dose of sterile saline injection was injected into t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com