Double function infusion protein for thrombolysis and anticoagulation , its preparation method and uses
A dual-efficiency, fusion protein technology, applied in chemical instruments and methods, peptide/protein components, animal/human proteins, etc., can solve problems such as adverse effects of curative effect stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
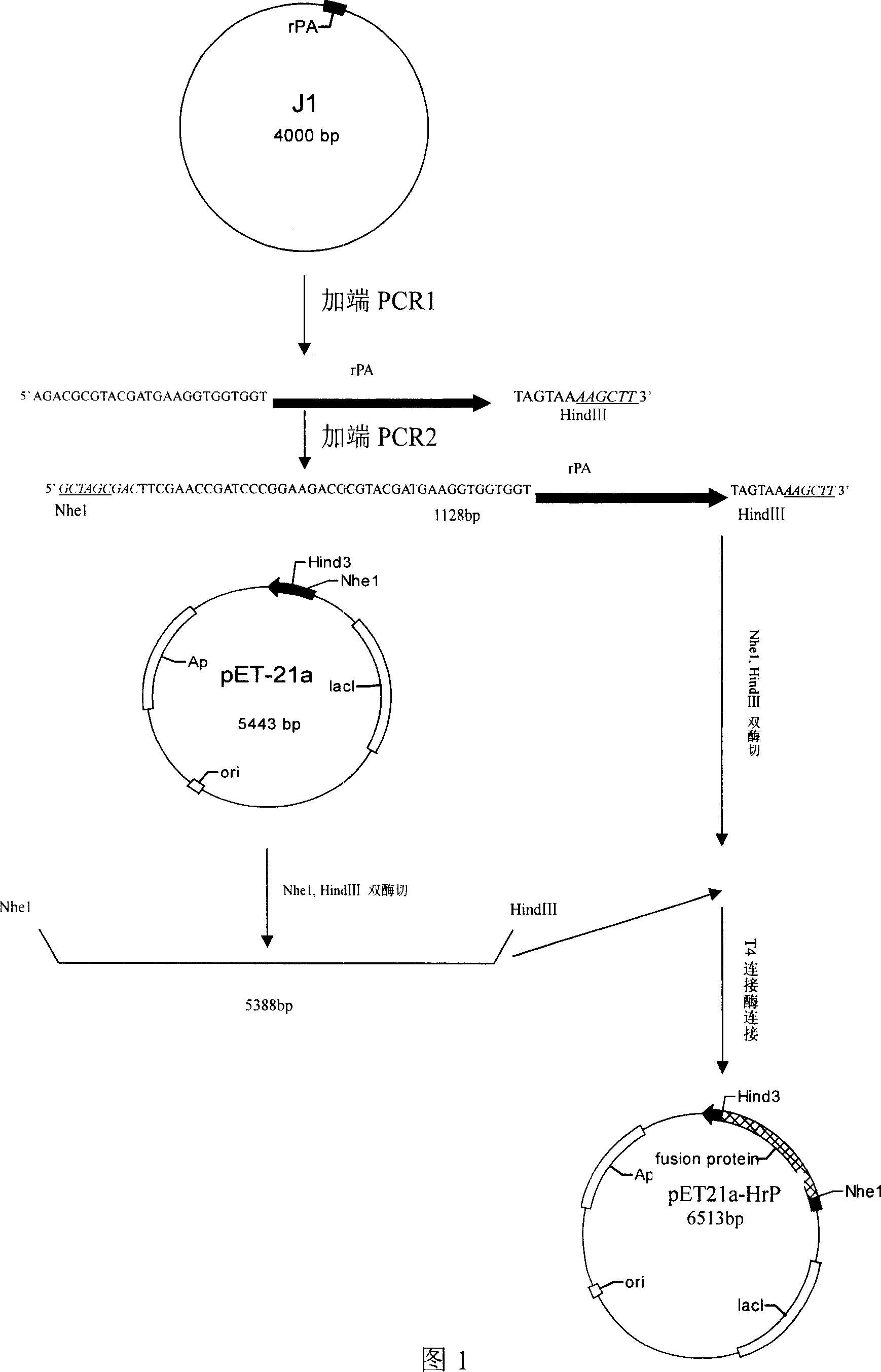
[0026] 1. Obtain the target gene sequence by PCR
[0027] As shown in Figure 1, according to the known gene sequence, apply the end-added PCR method in the r-PA gene (the J1 plasmid in Figure 1 contains the r-PA gene shown in SEQ ID NO.4, the aminoacid sequence of its coding Respectively as shown in SEQ ID NO.5 and SEQ ID NO.6), the 5' end adds 12 peptides (55-66) of recombinant hirudin HV3 and the gene of GlyGlyGly connecting peptide, but because the added end sequence two is greater than 60bp, it may The inaccuracy of the PCR results was caused, so the PCR was divided into two times in order to improve the accuracy of the PCR.
[0028] 1. The first round of PCR
[0029] Using the r-PA plasmid as a template and primers 1 and 2 as primers, TaqDNA polymerase catalyzed amplification to obtain the first-round PCR product P1.
[0030]Primer 1 (SEQ ID NO.10):
[0031] GGAGACGCGTACGATGAA GGTGGTGGT TCTTACCAAGGAAACAGTGAC
[0032] The underlined part is the connecting peptide sequ...
Embodiment 2
[0059] Example 2 Preparation and biological activity detection of fusion protein of hirudin 11 peptide and r-PA
[0060] The basic process is as in Example 1 above, except that the second round of PCR is different from Example 1, and all other operations and conditions are the same as Example 1.
[0061] Using P1 as a template and primers 4 and 5 as primers, TaqDNA polymerase catalyzed amplification to obtain the second-round PCR product P2.
[0062] Primer 4: (SEQ ID NO.13)
[0063] GGCTAGC TTCGAACCGATCCCGGAAGACGCGTACGATGAAGG
[0064] Among them, the boldface is the restriction site of NheI. The underlined part is the nucleotide sequence of hirudin III12 peptide.
[0065] Primer 5: (SEQ ID NO.14)
[0066] GC TTACTACGGTCGCATGTTGTCACGAATCCAGTCT
[0067] The shaded part is the restriction site of HindIII.
Embodiment 3
[0068] Example 3 Preparation and biological activity detection of fusion protein of hirudin 10 peptide and r-PA
[0069] The basic process is as in Example 1 above, except that the second round of PCR is different from Example 1, and all other operations and conditions are the same as Example 1.
[0070] Using P1 as a template and primers 6 and 5 as primers, TaqDNA polymerase catalyzed amplification to obtain the second-round PCR product P2.
[0071] Primer 6: (SEQ ID NO.15)
[0072] GGCTAGC GAACCGATCCCGGAAGACGCGTACGATGAAGG
[0073] Among them, the boldface is the restriction site of NheI. The underlined part is the nucleotide sequence of hirudin III12 peptide.
[0074] Primer 5: (SEQ ID NO.14)
[0075] GC TTACTACGGTCGCATGTTGTCACGAATCCAGTCT
[0076] The shaded part is the restriction site of HindIII.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com