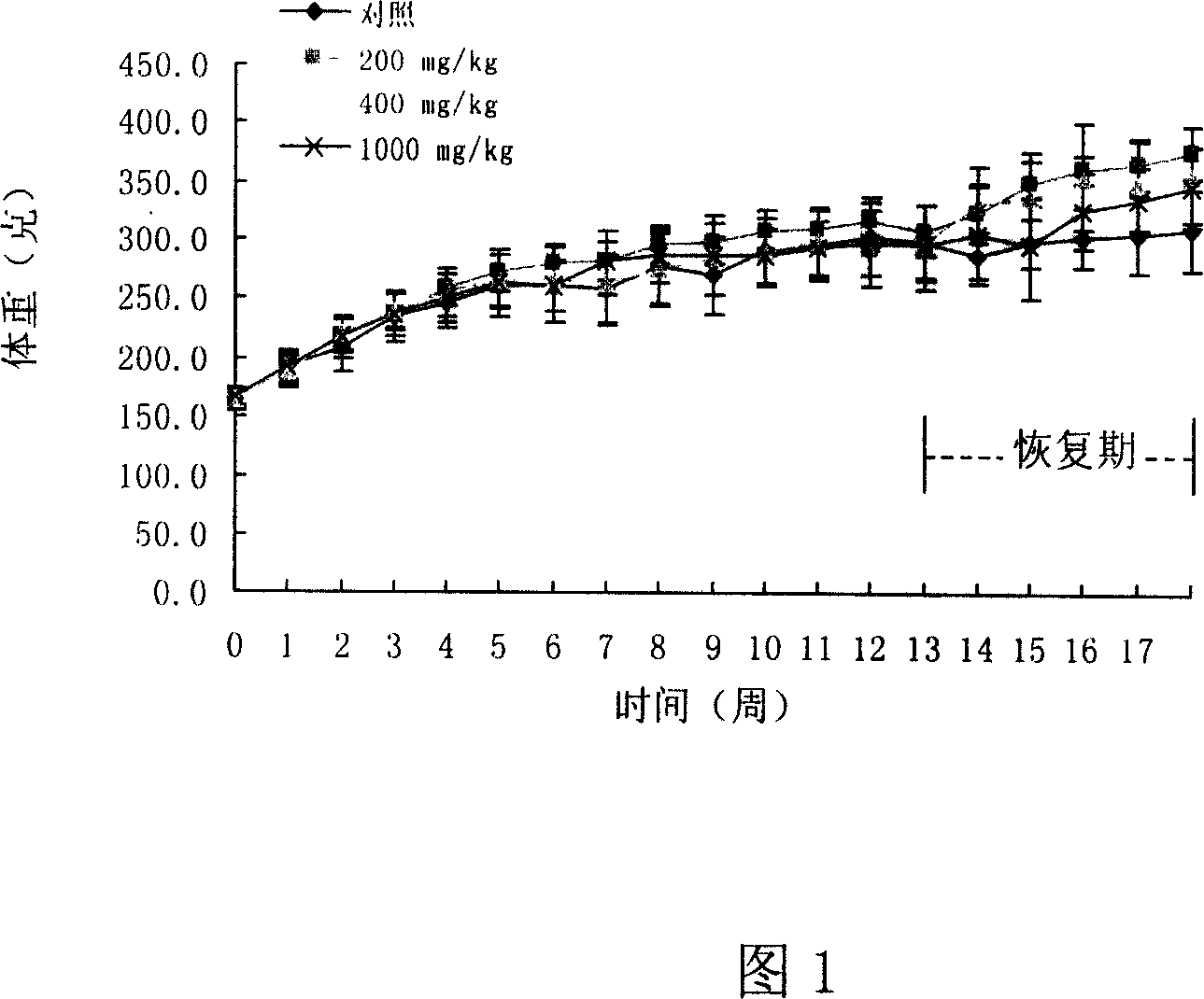
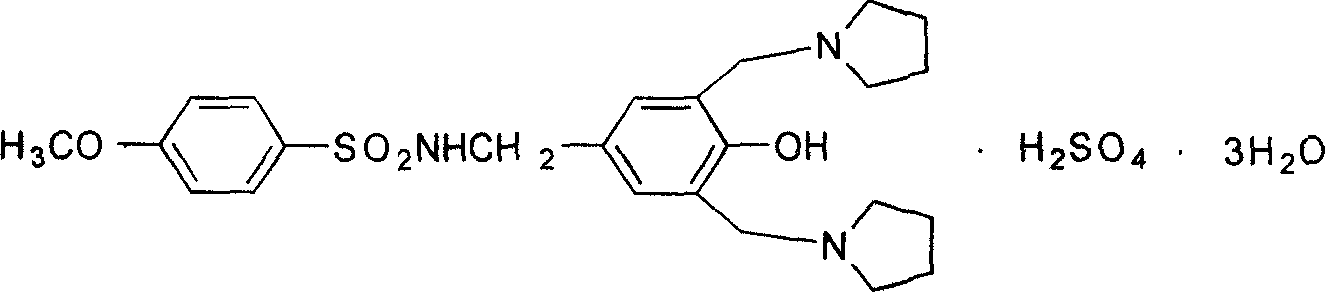
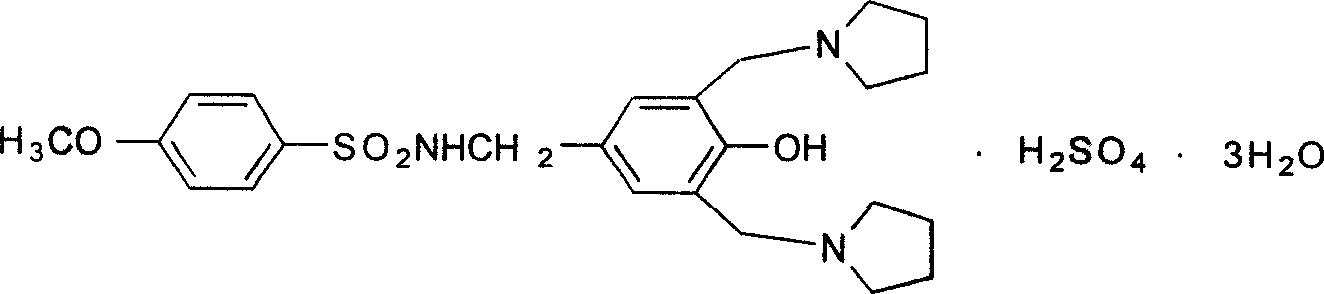
Schuqindin sulfate solid and target preparation and their making method
A technology of sulphuridine sulfate solid and sulphate sulphate target, which is applied in the field of pharmaceutical preparations, can solve problems such as preparation research that has not been reported in literature, and achieve the effects of improving therapeutic effect, prolonging action time, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of Shuxindine Sulfate Tablets
[0043] Take by weighing 200g of sulfindine sulfate and 20g of microcrystalline cellulose according to the prescription quantity, grind them finely respectively, cross 80 mesh sieves, after mixing uniformly by the equal amount incremental method, prepare soft material with starch slurry (5%) in an appropriate amount, and use 24 mesh Sieve and granulate, dry at 60°C for 3 hours, granulate with a 24-mesh sieve, add 0.66 g of magnesium stearate, mix well, and press into tablets.
[0044] 2. Dissolution Determination
[0045] Get 6 tablets of Suxindine Sulfate Tablets, and adopt the paddle method to carry out the dissolution test. Add 900mL of distilled water into the dissolution vessel, the temperature of the water bath is (37±0.5)°C, and the rotation speed is 50r / min. Operate according to the law, regularly (1, 5, 10, 15, 20, 30, 45min) sample 6ml (immediately add 6mL of distilled water), immediately filter through a 0.8μm filt...
Embodiment 2
[0048] In the embodiment 1, the experiment of influencing factor 3 of shuxindine sulfate tablet
[0049] According to the Chinese Pharmacopoeia 2000 edition two appendices (XIX C) pharmaceutical preparation stability key investigation project list to determine the investigation project of Suxindine sulfate tablet stability experiment is: character, content, related substance and dissolution rate.
[0050] 1 Lighting experiment
[0051] Get each 3 batch numbers of Suxindisulfate Tablet Tablets, put under the exposed fluorescent lamp (light intensity is 4500Lx), irradiate 10d, place 0,5,10d sampling, carry out to the items such as proterties, related substance, content, dissolution respectively Investigation and determination.
[0052] 2 high temperature test
[0053] Take 3 batches of Suxindine Sulfate Tablets, place them in a closed container under dry heat at 60°C for 10 days, take samples at 0, 5, and 10 days after placing them, and investigate and measure their properties...
Embodiment 3
[0059] Accelerated experiment and long-term experiment of Shuxindisulfate tablet in embodiment 1
[0060] 1 accelerated experiment
[0061] Take 3 batches of Suxindine sulfate tablets, place them at 40°C and RH75% for 6 months, take samples at 1, 2, and 3.6 months, and investigate the properties, related substances, content, and dissolution rate respectively.
[0062] 2 long-term experiments
[0063] Take 3 batches of Suxindine Sulfate Tablets, place them at 25°C and RH60% for 12 months, take samples at 0, 3, 6, 9, and 12 months, and conduct tests on their properties, related substances, content, and dissolution rate respectively. study.
[0064] Storage conditions
[0065] Accelerated experiments and long-term experiments showed that Suxindine Sulfate Tablets had good stability.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com