Preparation method of alumina
A technology of alumina and crude alumina, applied in the preparation of alumina/aluminum hydroxide, alumina/hydroxide, etc., can solve the problems of complex alumina process, high energy consumption, environmental and human harm, etc. The effect of sufficient source, simple process and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
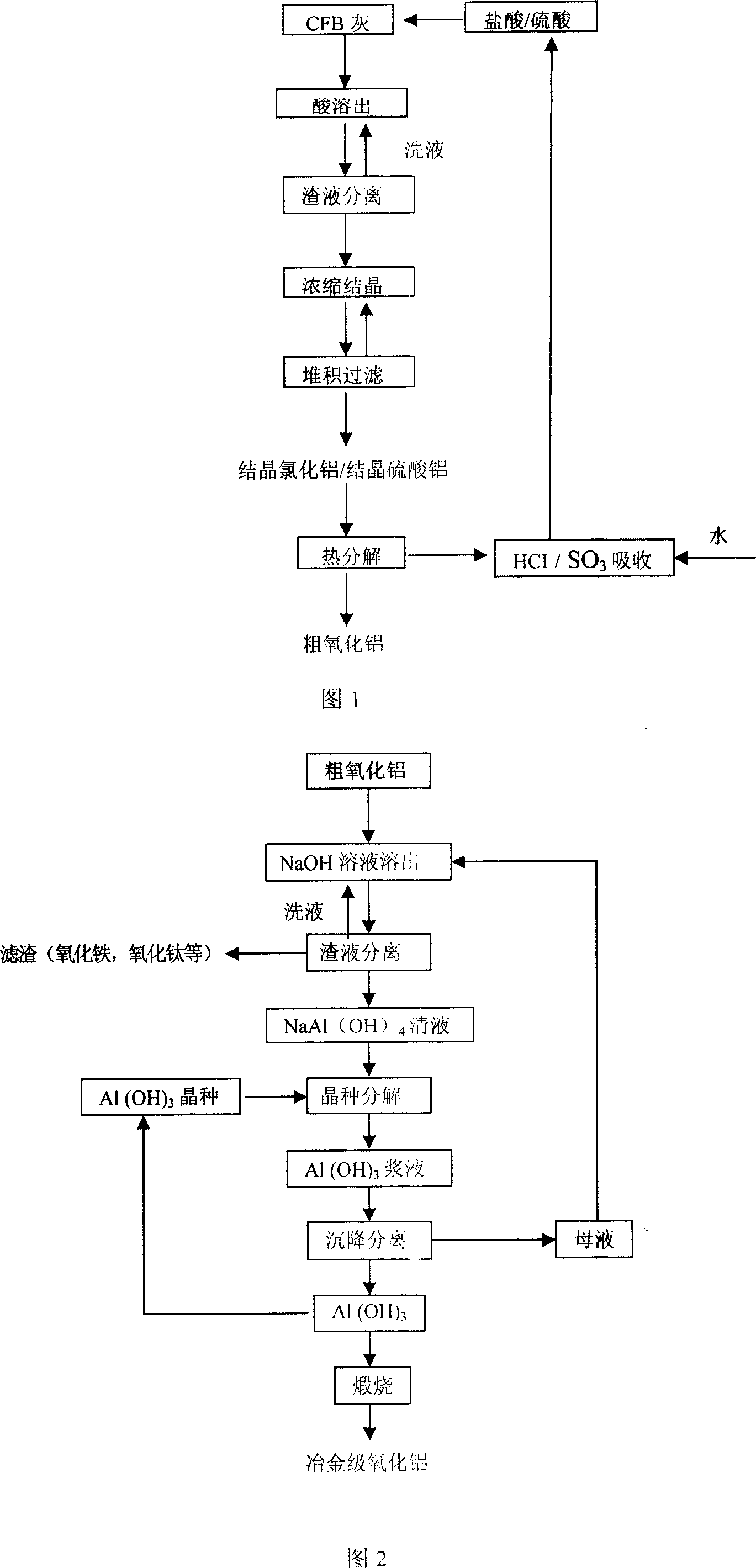
[0027] Take 1000 grams of circulating fluidized bed fly ash and place it in a reaction kettle, add 3000 ml of hydrochloric acid with a concentration of 22.5%, heat and stir, control the temperature at 100° C., and react for 2 hours. After the acid-dissolution reaction is completed, the residue liquid is separated, and the filtrate is used for the next step of concentration and crystallization, and the filter residue is washed with water, and the washing liquid is returned to the acid-dissolution reaction for reuse. The aluminum chloride clear liquid after the slag liquid separation is sent to the concentration tank for concentration and crystallization, and the precipitated crystalline aluminum chloride is separated by heap filtration. The obtained crystalline aluminum chloride is heated to 500°C, thermally decomposed to obtain crude alumina, and the hydrogen chloride gas produced by pyrolysis is absorbed by water and returned to the acid solution reaction solution for reuse. ...
Embodiment 2
[0030] Take 1000 grams of circulating fluidized bed fly ash and place it in a reaction kettle, add 2500 ml of hydrochloric acid with a concentration of 30%, heat and stir, control the temperature at 90° C., and react for 2 hours. After the acid-dissolution reaction is completed, the residue liquid is separated, and the filtrate is used for the next step of concentration and crystallization, and the filter residue is washed with water, and the washing liquid is returned to the acid-dissolution reaction for reuse. The aluminum chloride clear liquid after the slag liquid separation is sent to the concentration tank for concentration and crystallization, and the precipitated crystalline aluminum chloride is separated by heap filtration. The obtained crystalline aluminum chloride is heated to 450°C, thermally decomposed to obtain crude alumina, and the hydrogen chloride gas produced by pyrolysis is absorbed by water and returned to the acid solution reaction solution for reuse.
[...
Embodiment 3
[0033] Take 1000 grams of circulating fluidized bed fly ash and place it in a reactor, add 3000 ml of sulfuric acid with a concentration of 40%, heat and stir, control the temperature at 100°C, and react for 1.5 hours. After the acid dissolution reaction, the ore pulp is separated from the slag liquid, and the filtrate is used for the next step of concentration and crystallization. The filter residue is washed with water, and the washing liquid is returned to the acid dissolution reaction for reuse. The aluminum sulfate separated from the slag liquid is sent to the concentration tank at night for concentration and crystallization, and the precipitated crystalline aluminum sulfate is separated by heap filtration. The obtained crystalline aluminum sulfate is heated to 500°C, thermally decomposed to obtain crude alumina, and the sulfur trioxide gas produced by pyrolysis is absorbed by water and returned to the acid solution reaction solution for reuse.
[0034] Grind the coarse a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com