Preparations stabilized over long time
A preparation and stable technology, applied in the field of G-CSF preparations, can solve problems such as contamination, complex virus removal, deterioration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
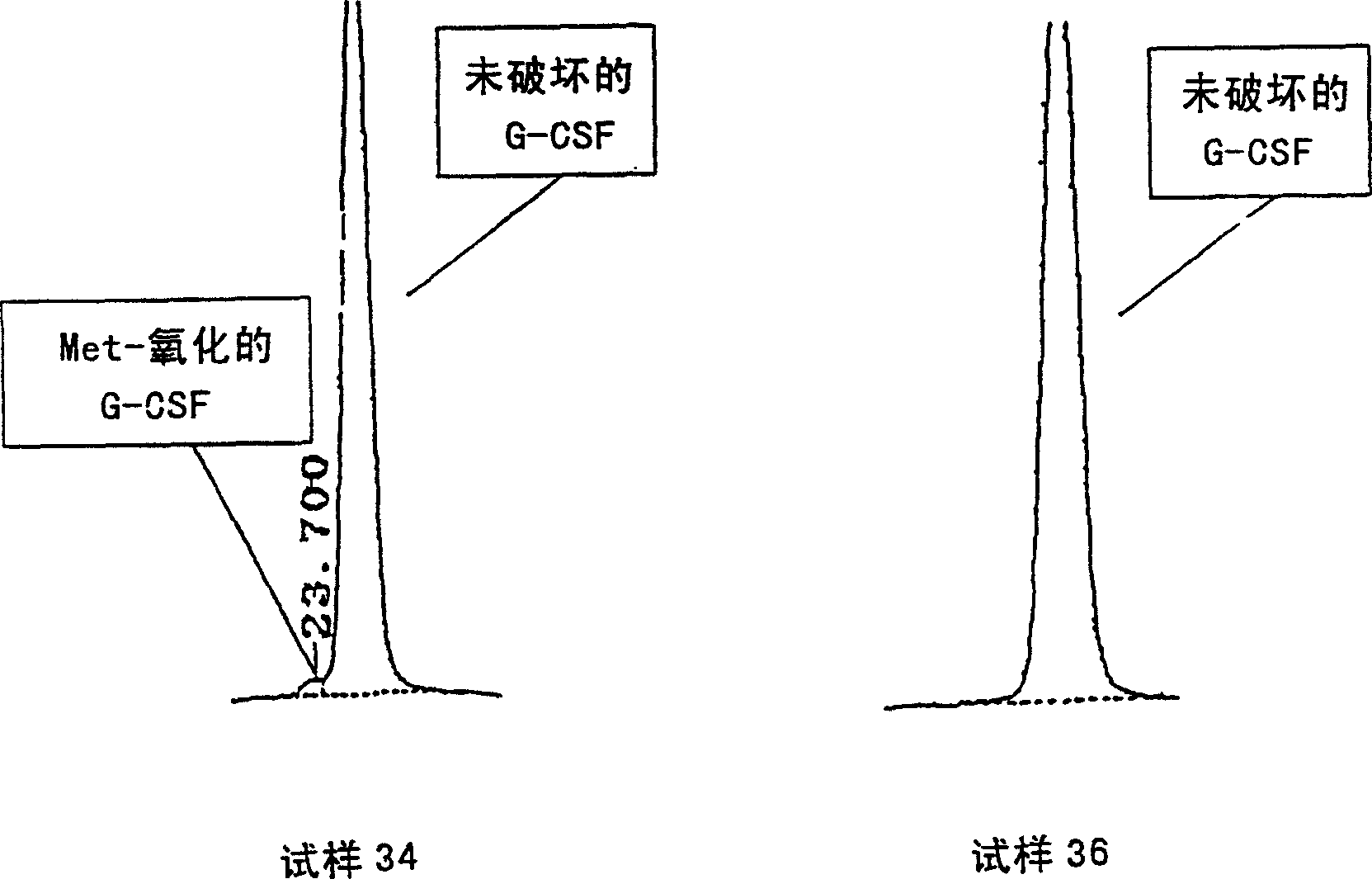
[0093] The effect of pH value on the remaining rate of G-CSF
[0094] Calculate the residual rate of G-CSF in samples 1 and 2 prepared according to different pH values in Table 1 after the accelerated test at 60°C for 2 weeks and 50°C for 1 month according to the equation in method 1. The results are shown in Table 3.
[0095] Sample 1, pH7.4
[0096] As shown in the table, the formulation at pH 6.5 is more stable than that at pH 7.4.
Embodiment 2
[0098] Effects of Various Amino Acids on G-CSF Surplus Rate (1)
[0099] Prepare samples 3-6 (containing G-CSF 100 μg) and samples 7-10 (containing G-CSF 250 μg) according to the amino acids listed in Table 1, and calculate according to the equation in method 1. Accelerated test at 60°C for 2 weeks and 50°C for 1 month The remaining rate of G-CSF in the above preparations. The results are shown in Tables 4 and 5.
[0100] Sample 3
[0101] Sample 7
[0102] The addition of phenylalanine or arginine alone improved stability at any G-CSF content compared to formulations without amino acids, but not significantly. Adding phenylalanine and arginine at the same time can significantly improve the stability of the preparation.
Embodiment 3
[0104] Effects of Various Amino Acids on G-CSF Surplus Rate (2)
[0105] According to the amino acids listed in Table 1, prepare samples 11-20 (containing phenylalanine as amino acid 1, and lysine, histidine, arginine, aspartic acid, glutamic acid, serine, threonine , tyrosine, asparagine and glutamine as amino acid 2), and samples 21-33 (containing arginine as amino acid 1, and alanine, valine, leucine, isoleucine acid, methionine, tryptophan, phenylalanine, proline, glycine, serine, threonine, asparagine and glutamine as the amino acid 2), calculated according to the equation in method 160 The residual rate of G-CSF in the above preparations after 2 weeks at ℃ and 1 month at 50℃. The results are shown in Tables 6 and 7.
[0106] 50℃, January
[0107] 50℃, January
[0108] It can be observed that phenylalanine and lysine, phenylalanine and histidine, phenylalanine and arginine, phenylalanine and aspartic acid, phenylalanine and gluta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com