Quality control method of kidney-replenishing blood-nourishing soft capsule
A quality control method and soft capsule technology, which can be used in capsule delivery, medical preparations containing active ingredients, blood diseases, etc., and can solve problems such as cumbersome processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0140] Embodiment 1: the quality control method of Bushen Yangxue Soft Capsules
[0141] Weigh 480g Polygonum multiflorum, 120g Angelica, 240g Black Bean, 120g Achyranthes bidentata (salt), 120g Poria, 120g Cuscuta, 60g Psoralen (salt), 120g Lycium barbarum according to the prescription, and add 8 times the amount of the above eight herbs Extract with 70% ethanol three times, each time for 1.5 hours, combine the decoction, filter, and recover the filtrate from ethanol to obtain a clear ointment for later use; add 10 times the amount of water to decoct the medicinal residue twice, each time for 1 hour, filter, and recover the filtrate for later use . Combine the above two clear pastes, continue to concentrate to the clear paste with a relative density of 1.25 (50°C), spray dry, collect the dry powder, pass through a 120 mesh sieve, add vegetable oil, lecithin, beeswax and other auxiliary materials, stir well, and press 1000 soft capsules Grains, that is.
[0142] 10 batches o...
Embodiment 2
[0161] Embodiment 2: the quality control method of Bushen Yangxue Soft Capsules
[0162] The kidney-tonifying and blood-reinforcing soft capsules were prepared by the same preparation method as in Example 1.
[0163] 1. Determination according to the following high performance liquid chromatography:
[0164] Chromatographic conditions: the mobile phase acetonitrile-water volume ratio is 20:80; the detection wavelength is 320nm.
[0165] Preparation of the reference solution: the same as in Example 1 to prepare a solution with a concentration of 60 μg / ml.
[0166] The preparation of need testing solution: get this product content, mix, get about 0.50g, weigh accurately, all the other are with embodiment 1.
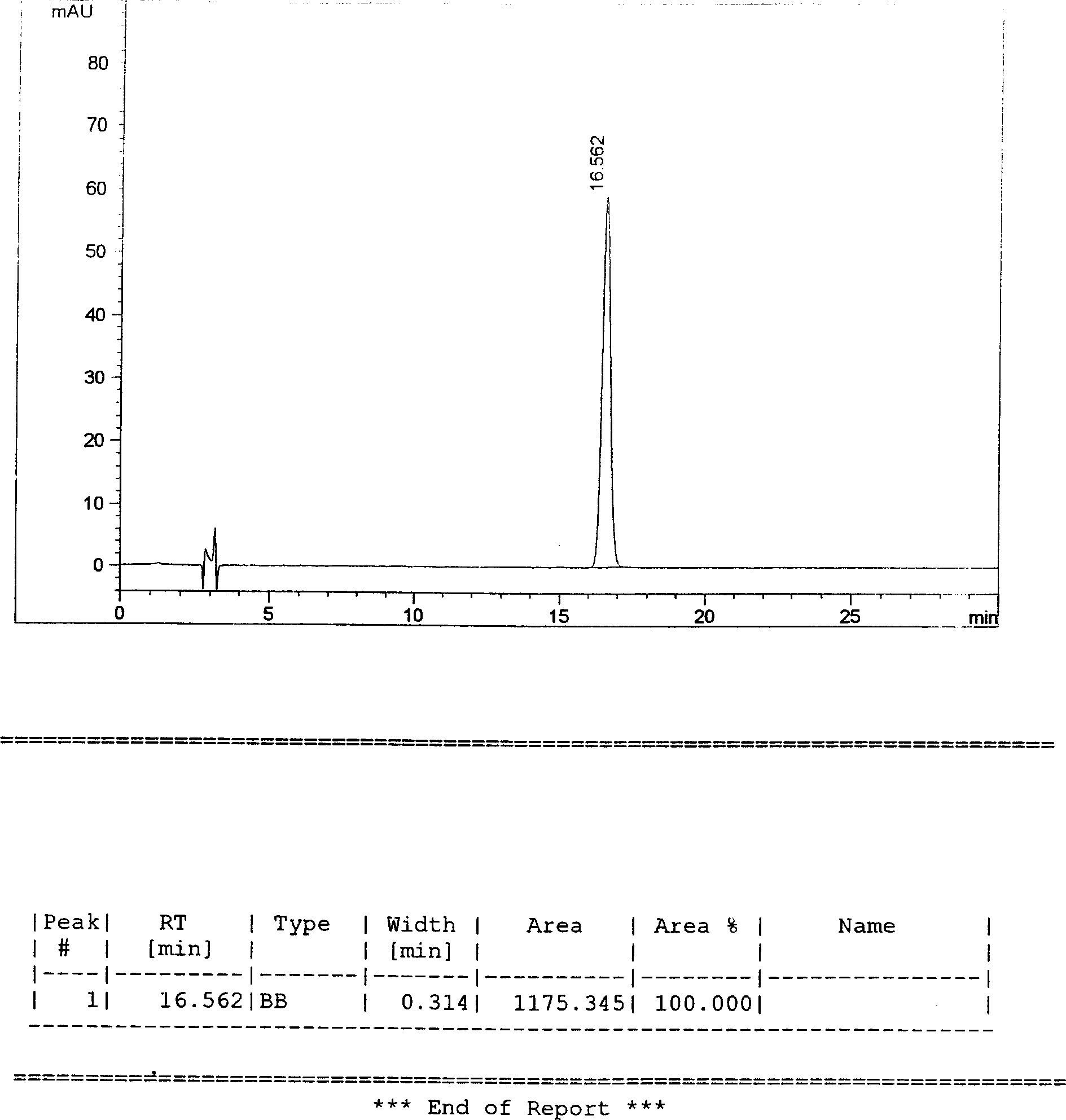
[0167] Determination method: the same as in Example 1, the calculated result is that the content of stilbene glycoside is 3.2mg / 0.65g.
[0168] 2. Identification by TLC
[0169] TLC Identification of Radix Polygoni Multiflori
[0170] (1) Preparation of the test soluti...
Embodiment 3
[0177] Embodiment 3: the quality control method of Bushen Yangxue Soft Capsules
[0178] The kidney-tonifying and blood-reinforcing soft capsules were prepared by the same preparation method as in Example 1.
[0179] 1. Determination according to the following high performance liquid chromatography
[0180] Chromatographic conditions and system suitability test: use octadecylsilane bonded silica gel as filler; mobile phase acetonitrile-water volume ratio 22:78; detection wavelength is 322nm.
[0181] Preparation of the reference solution: same as in Example 1, configured into a solution with a concentration of 10 μg / ml.
[0182] The preparation of need testing solution: get this product content 0.05g, with embodiment 1.
[0183] Assay method: same as in Example 1, the calculated stilbene glycoside content is 4.1mg / 0.65g.
[0184] 2. Identification by TLC
[0185] TLC identification of Radix Polygoni Multiflori:
[0186] (1) Preparation of the test solution Get about 0.5g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com