Conjugated polymer containing sulfonic group substituted triarylamine and use thereof
A conjugated polymer and triarylamine technology, applied in the field of organic compounds, can solve the problems that are not involved in preparation, application limitation of cross-linked hole transport materials, difficult initiator impurities, etc., and achieve good thermal stability and electrochemical stability. properties, the device fabrication process is simple and feasible, and the effect of excellent hole transport
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
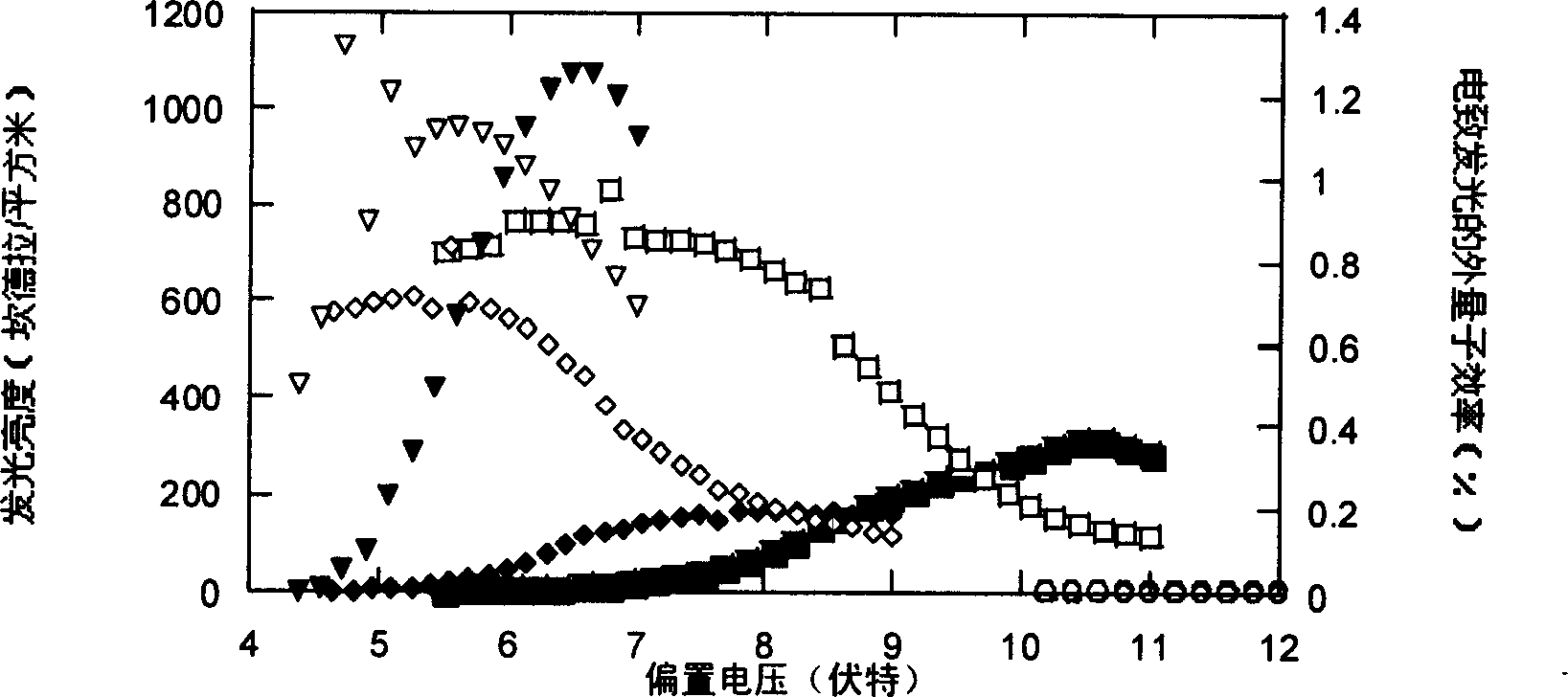
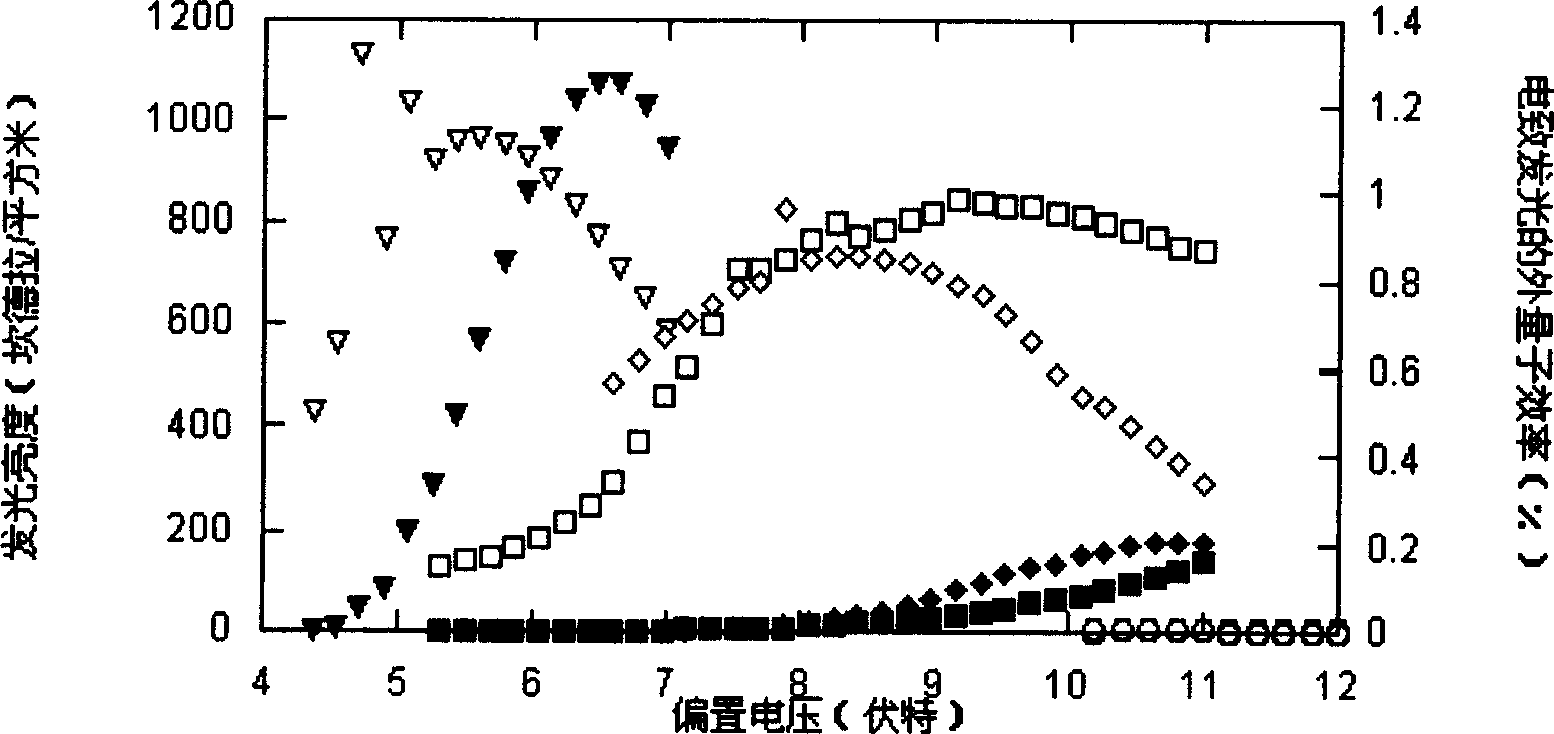
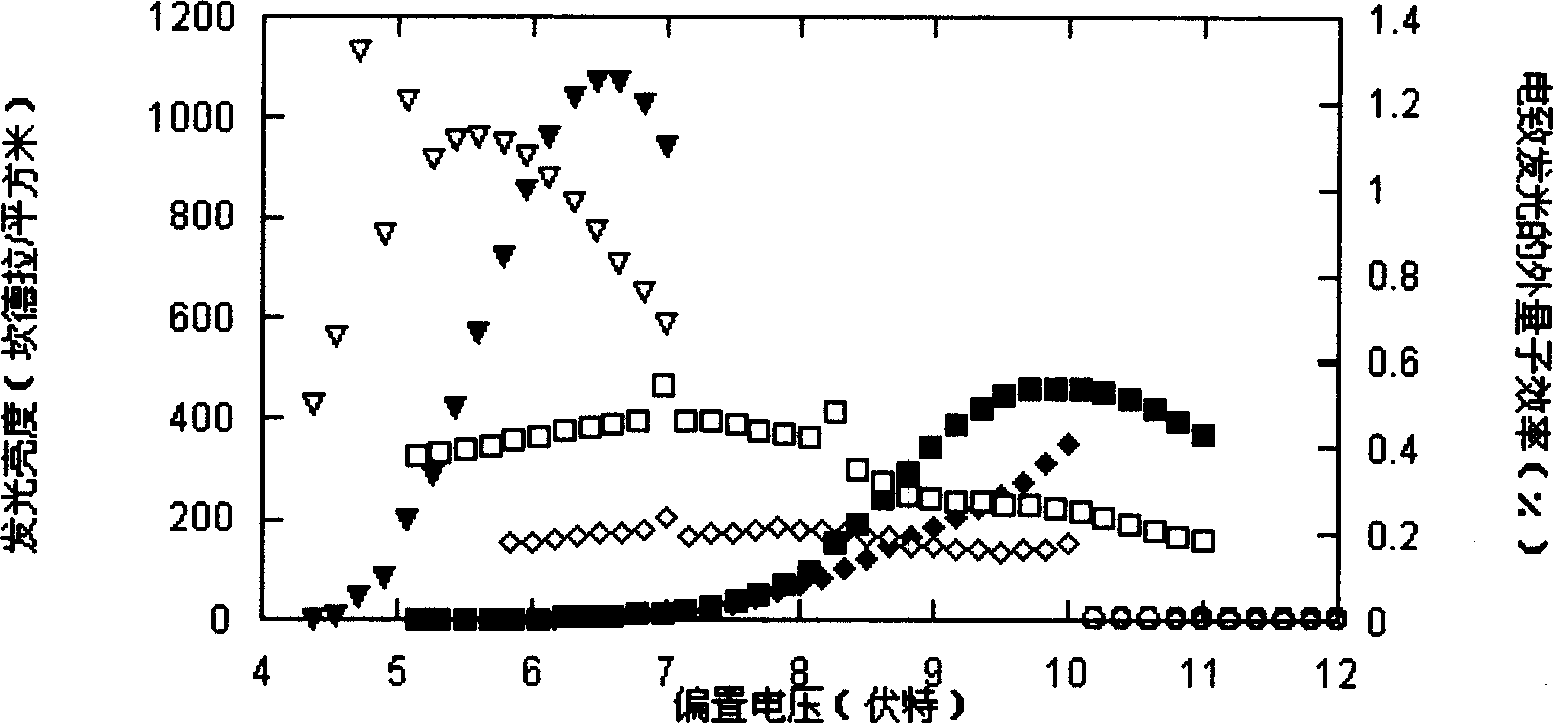
Image
Examples
Embodiment 1
[0074] Embodiment 1: preparation 4,4'-dibromotriphenylamine
[0075] 4,4'-Dibromotriphenylamine was prepared according to the method published in "Syn. Met" (Syn. Met) 122 (2001) 363. Weigh triphenylamine (4.9 g, 20 mmol) and measure N, N-dimethylformamide (DMF) 100 ml into a 250 ml three-necked flask, and dissolve completely after stirring, and add to this solution at room temperature N-bromosuccinimide (NBS) (7.12 g, 40 mmol) was added to it, and after two days of reaction at room temperature, the solvent was evaporated under reduced pressure below 70°C, and column separation was carried out after extraction with water and dichloromethane. After distilling off the solvent, 7.9 g of the product was obtained, a yield of 98%. 1HNMR, 13CNMR and GC-MASS tests show that it is the target product 4,4'-dibromotriphenylamine. Its chemical reaction equation is as follows:
[0076]
Embodiment 2
[0077] Embodiment 2: Preparation has the triarylamine compound of substituent
[0078] Preparation of triarylamine compounds with substituents Take the preparation of N,N-diphenyl-(4-trifluoromethyl)aniline as an example, and prepare N,N-diphenyl according to the method disclosed in the world patent WO 01 66618 in 2000 -(4-Trifluoromethyl)aniline. Weigh into diphenylamine (15.57 grams, 92 mmoles) in the there-necked flask of 500 milliliters, (4-trifluoromethyl) iodobenzene (31.43 grams, 115 mmoles), palladium acetate (404 mgs, 1.8 mmoles), Tri-o-cresylphosphine (1.19 g, 3.9 mmol), sodium tert-butoxide (12.50 g, 130 mmol) and dry purified toluene (200 mL). The suspension was stirred at reflux for 12 hours under an inert atmosphere. Thereafter, it was filtered, and the solvent was distilled off, followed by column separation using toluene as an eluent. After distilling off the solvent, 23.16 g of the product were obtained, a yield of 80%. 1HNMR, 13CNMR and GC-MASS tests show...
Embodiment 3
[0081] Embodiment 3: Preparation of dibromo-substituted triarylamine compound
[0082] Preparation of dibromo-substituted triarylamine compound Taking N,N-di-p-bromophenyl-(4-trifluoromethyl)aniline as an example, it was prepared according to the method disclosed in the world patent WO 01 66618 in 2000. Under nitrogen protection, N, N-diphenyl-(4-trifluoromethyl)aniline (23.16 g, 74 mmol) was weighed and dissolved in 200 ml of anhydrous DMF, and NBS ( 27.6 g, 155 mmol) in 200 mL of DMF and keeping the internal temperature of the reaction below 20 °C. After 1 hour the reaction was complete and it was poured into 3 liters of water. The crude product precipitated out, was filtered and recrystallized several times from petroleum ether-diethyl ether (volume ratio 80 / 100) to give the product 15.2 g. 1HNMR, 13CNMR and GC-MASS tests showed that it was the target product N,N-di-p-bromophenyl-(4-trifluoromethyl)aniline. Its chemical reaction equation is as follows:
[0083]
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com