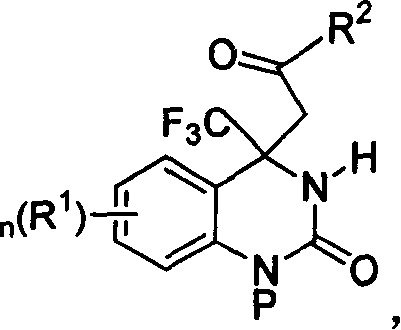
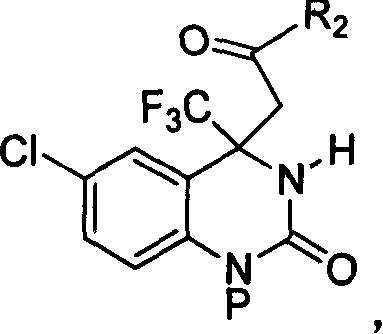
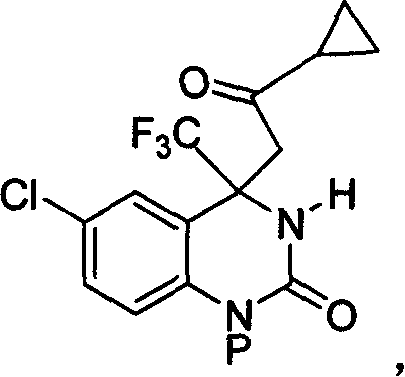
4,4-disubstituted-3,4-dihydro-2(1H)- quinolones and synthesis process and use thereof
A ketone compound, compound technology, applied in 4 fields, can solve problems such as harsh process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Preparation of (+ / -)-6-chloro-4-(2-oxopropyl)-4-trifluoromethyl-3,4-dihydro-2(1H)-quinazolinone (R 2 = methyl)
[0061]
[0062] At room temperature, 250 mg (1 mmol) of compound 1 was dissolved in a mixed solution of 2 ml of acetone and 8 ml of anhydrous DMSO, and 23 mg (0.2 mmol) of racemic proline was added. The reaction can be tracked by TLC (petroleum ether: ethyl acetate 1:1), and can also be judged according to the color of the reaction system (from yellow to colorless), and the reaction is complete within 1 h. Add 10ml of water, extract with 10ml*3 ethyl acetate, combine the extracts, spin off the solvent, perform flash column chromatography (petroleum ether: ethyl acetate 2:1), and obtain 300mg (quantitative) of compound 2.
[0063] 1 HNMR (300MHz, acetone-d6 (deuterated acetone)) δ: 9.09 (br, s, NH), 7.11 (br, s, NH), 7.41 (s, 1H), 7.31 (dd, 1H, J=9, J=2), 6.98(d, 1H, J=9), 3.80(d, 1H, J=18), 3.47(d, 1H, J=18), 2.14(s, 3H);
[0064] 13 CNMR (75MHz, DMSO...
Embodiment 2
[0069] (+ / -)1-(4-methoxybenzyl)-6-chloro-4-(2-oxopropyl)-4-trifluoromethyl-3,4-dihydro-2(1H )-The preparation of quinazolinone (R 2 = methyl, P = 4-methoxybenzyl)
[0070]
[0071] At room temperature, 370 mg (1 mmol) of compound 1 was dissolved in a mixed solution of 2 ml of acetone and 8 ml of anhydrous DMSO, and 23 mg (0.2 mmol) of racemic proline was added. The reaction can be tracked by TLC (petroleum ether: ethyl acetate 3:1), and can also be judged according to the color of the reaction system (from bright yellow to colorless), and the reaction is complete within 2 hours. Add 10ml of water, extract with 10ml*3 ethyl acetate, combine the extracts, spin off the solvent, perform flash column chromatography (petroleum ether: ethyl acetate 3:1), and obtain 420mg, (99%) of compound 2a.
[0072] 1 HNMR (300MHz, CDCl3) δ: 7.16-7.20(m, 4H), 6.84-6.89(m, 3H), 6.76(d, 1H, J=9), 5.11(s, 2H), 3.77(s, 3H) , 3.33(d, 1H, J=17), 3.16(d, 1H, J=17), 2.20(s, 3H);
[0073] 13 CNMR ...
Embodiment 3
[0078] Preparation of (+ / -)-6-chloro-4-(2-oxobutyl)-4-trifluoromethyl-3,4-dihydro-2(1H)-quinazolinone (R 2 = ethyl)
[0079]
[0080] At room temperature, 250 mg (1 mmol) of compound 1 was dissolved in a mixed solution of 2 ml butanone and 8 ml anhydrous DMSO, and 23 mg (0.2 mmol) of racemic proline was added. The reaction can be tracked by TLC (petroleum ether: ethyl acetate 1:1), and can also be judged according to the color of the reaction system (from yellow to colorless), and the reaction is complete within 24 hours. Add 10ml of water, extract with 10ml*3 ethyl acetate, combine the extracts, spin off the solvent, perform flash column chromatography (petroleum ether: ethyl acetate 2:1), and obtain 280mg (85%) of compound 3.
[0081] 1 HNMR (300MHz, acetone-d6) δ: 8.93 (br s, NH), 7.19 (br s, NH), 7.41 (s, 1H), 7.31 (dd, 1H, J=9, J=2), 6.98 ( d, 1H, J=8), 3.75(d, 1H, J=18.0), 3.48(d, 1H, J=18), 2.52(m, 2H), 0.907(t, 3H, J=7);
[0082] 13 CNMR (75MHz, DMSO-d6) δ: 204...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com