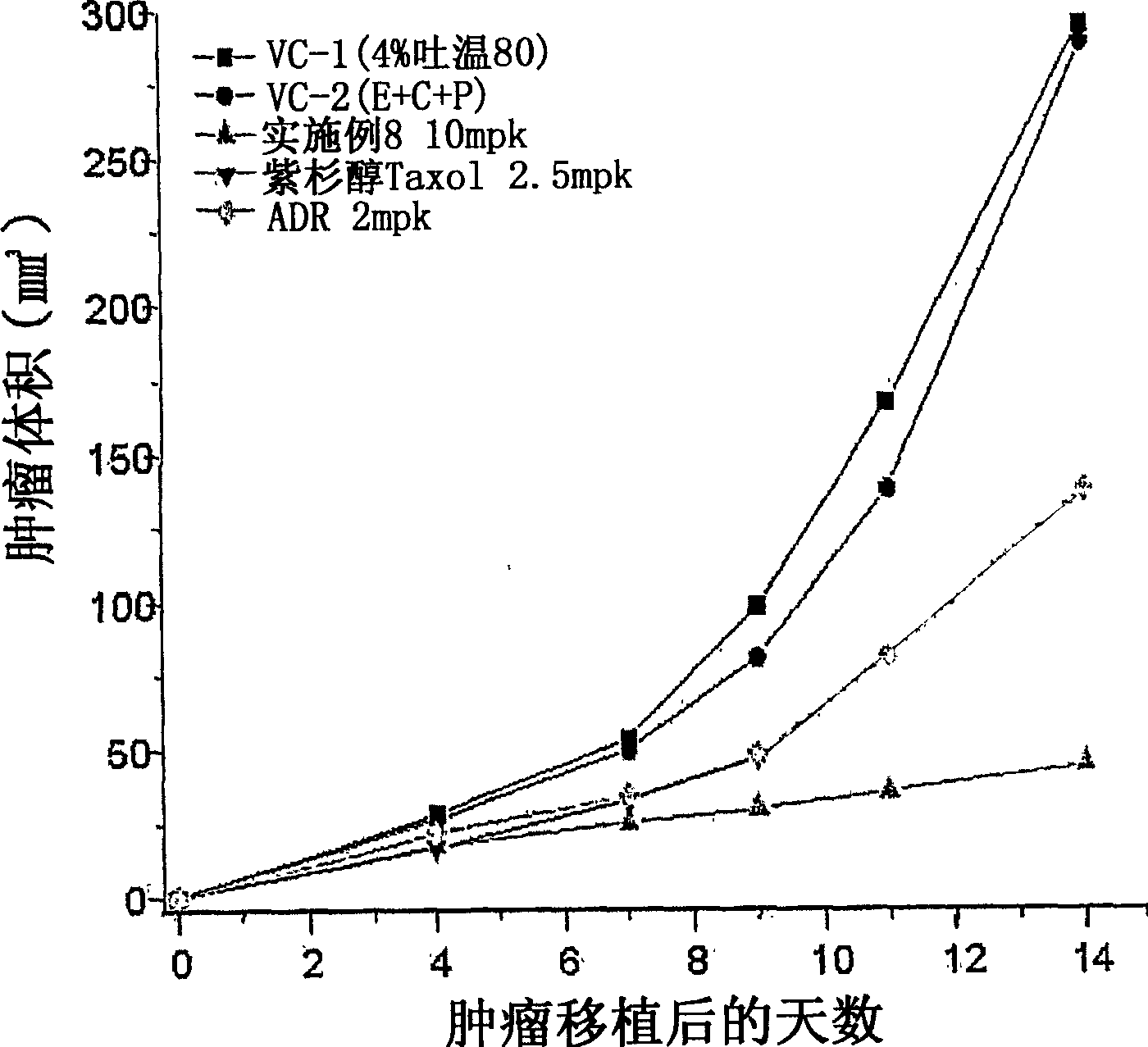
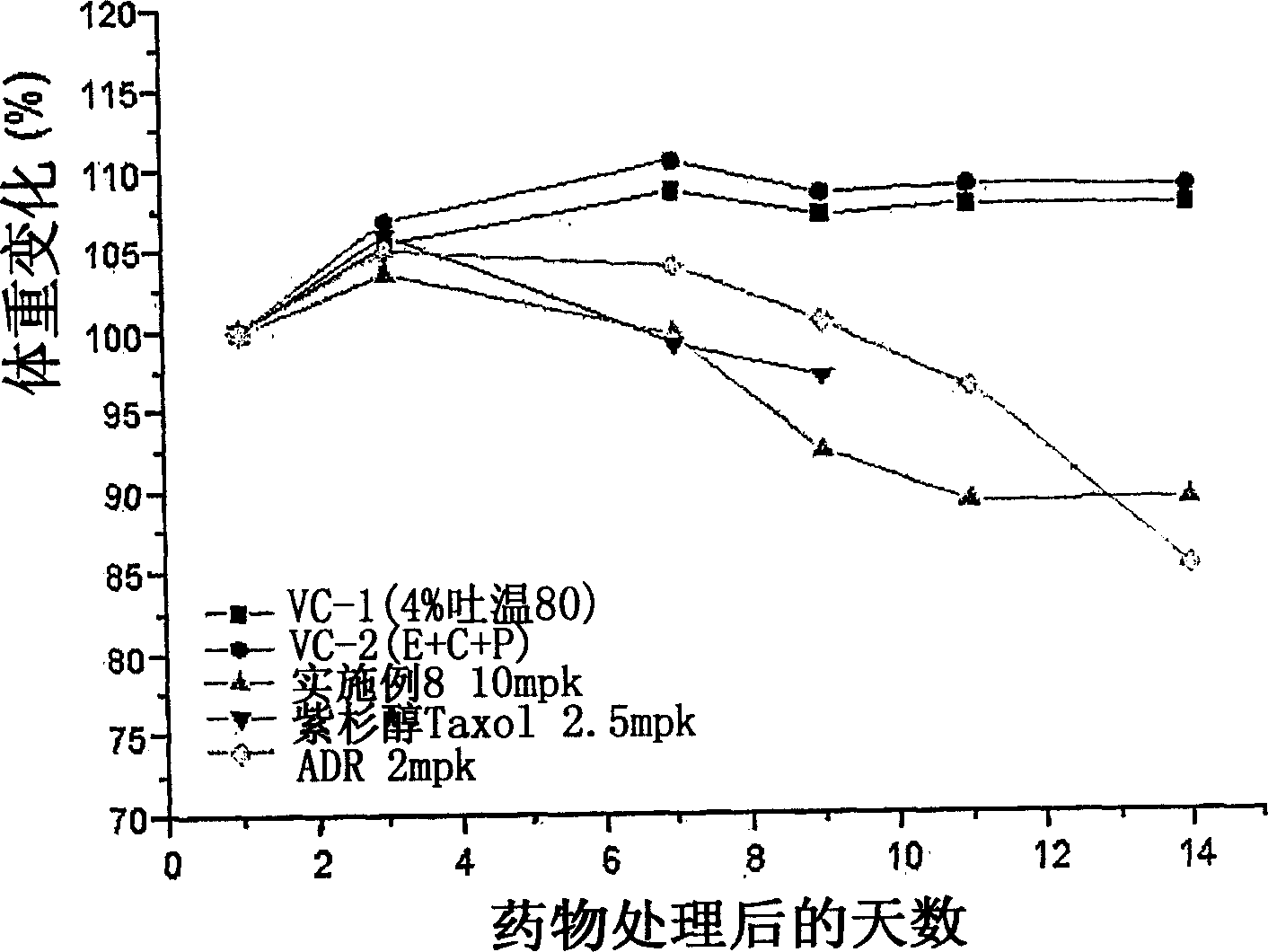
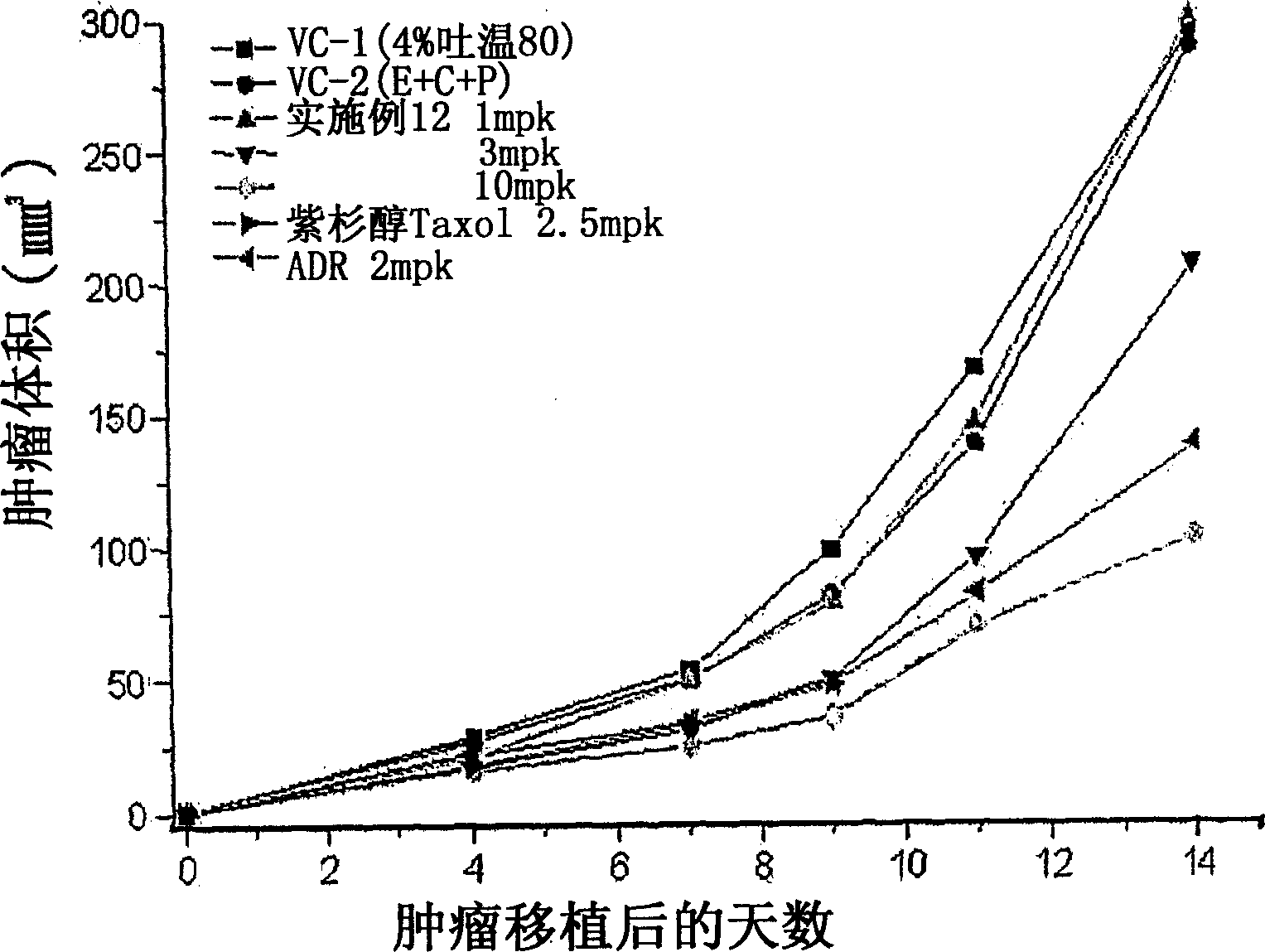
Tricyclic derivatives or pharmaceutically acceptable salts thereof, their preparations and pharmaceutical compositions containing them
A derivative and pharmaceutical technology, applied in the direction of drug combination, preparation of organic compounds, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0175] 6-Nitrooxymethyl-N-[(7S)-1,2,3-trimethoxy-10-methylthio-9-oxo-5,6,7,9-tetrahydro-benzo[ a] geng Preparation of en-7-yl]-nicotinamide
[0176] 6-Hydroxymethyl-N-[(7S)-1,2,3-trimethoxy-10-methylthio-9-oxo-5,6,7,9-tetrahydro-benzene and [a] Preparation of hepten-7-yl]-nicotinamide
[0177]
[0178] 6-Hydroxymethyl-nicotinic acid was synthesized according to the method described in (Bioorg. Med. Chem. Lett, 1996, 6, 3025-3028).
[0179] EDCI (308 mg, 1.60 mmol) was added to 7-amino-1,2,3-trimethoxy-10-methylthio-6,7-dihydro-5H-benzo[a]-heptanyl at 0 °C Capten-9-one (300 mg, 0.80 mmol), 6-hydroxymethylnicotinic acid (135 mg, 0.88 mmol) and DMAP (60 mg, 0.48 mmol) were dissolved in a solution of 10 ml of acetonitrile. The reaction mixture was stirred at room temperature for 2 hours. Water was added to terminate the reaction, and the aqueous layer was extracted with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulfate, filtered a...
Embodiment 2~4
[0186] Compounds of Example 2-Example 4 were synthesized in a similar manner to that described in Example 1, and intermediates were prepared by the methods described below.
[0187] Preparation of 5-hydroxymethyl-furan-2-carboxylic acid
[0188] 5-Hydroxymethyl-furan-2-carboxylic acid was synthesized following the method described in (Helv. Chim. Acta, 1926, 9, 1068).
[0189] Preparation of 3-hydroxymethylbenzoic acid
[0190]
[0191] Diethyl isophthalate (9.100 g, 40.95 mmol) was dissolved in tetrahydrofuran (20 mL). Then, lithium borohydride (11.26 ml, 22.52 mmol, 2M solution in tetrahydrofuran) was slowly added dropwise thereto, and the reaction mixture was refluxed for 3 hours. Water was added to terminate the reaction, and the aqueous layer was extracted with ethyl acetate. The combined organic layers were dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (hexane:ethyl...
Embodiment 2
[0194] Example 2: 5-Nitrooxymethyl-furan-2-carboxylic acid-[(7S)-1,2,3-trimethoxy-10-methylthio-9-oxo -5,6,7,9-Tetrahydro-benzo[a]heptalten-7-yl]-amide
[0195]
[0196] 1 H NMR (400MHz, CDCl 3 ): δ2.37-2.51(m, 3H), 2.46(s, 3H), 2.61-2.92(m, 1H), 3.74(s, 3H), 3.93(s, 3H), 3.98(s, 3H), 4.82-4.85(m, 1H), 4.85(d, J=13.2Hz, 1H), 4.90(d, J=13.2Hz, 1H), 6.39(s, 1H), 6.58(s, 1H), 6.64(s , 1H), 7.16(d, J=10.6Hz, 1H), 7.42(d, J=10.2Hz, 1H), 7.72(s, 1H), 8.99(s, 1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com