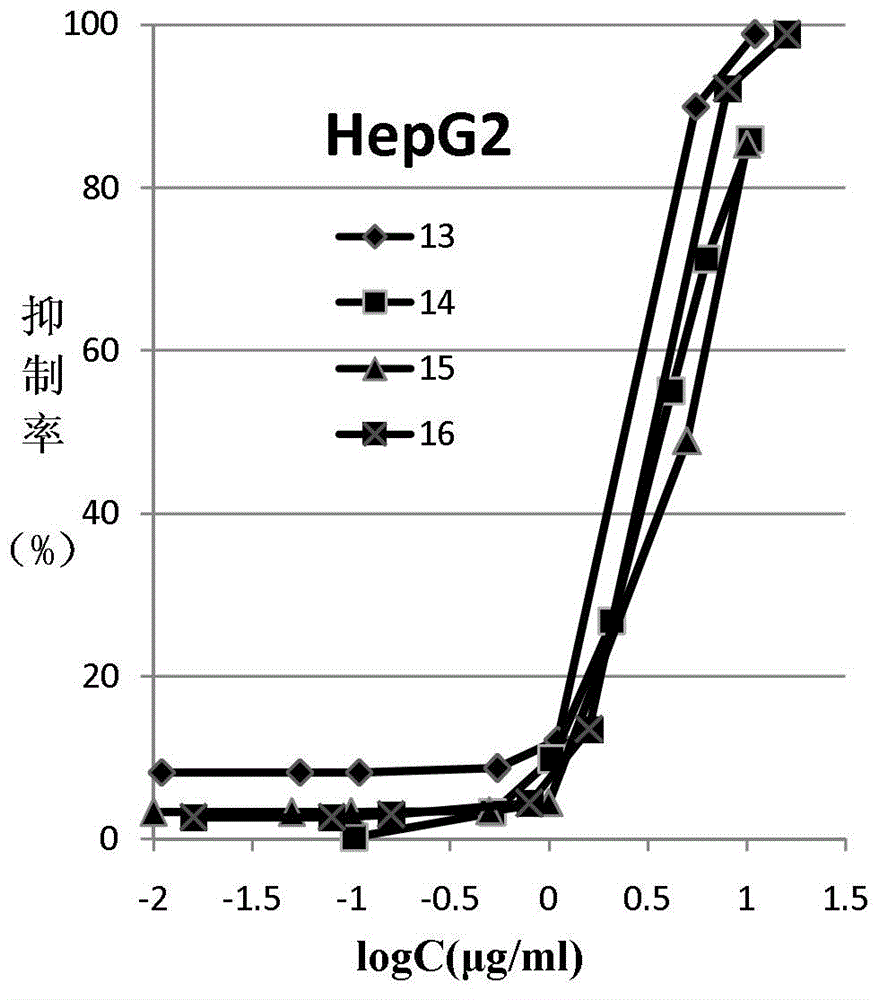
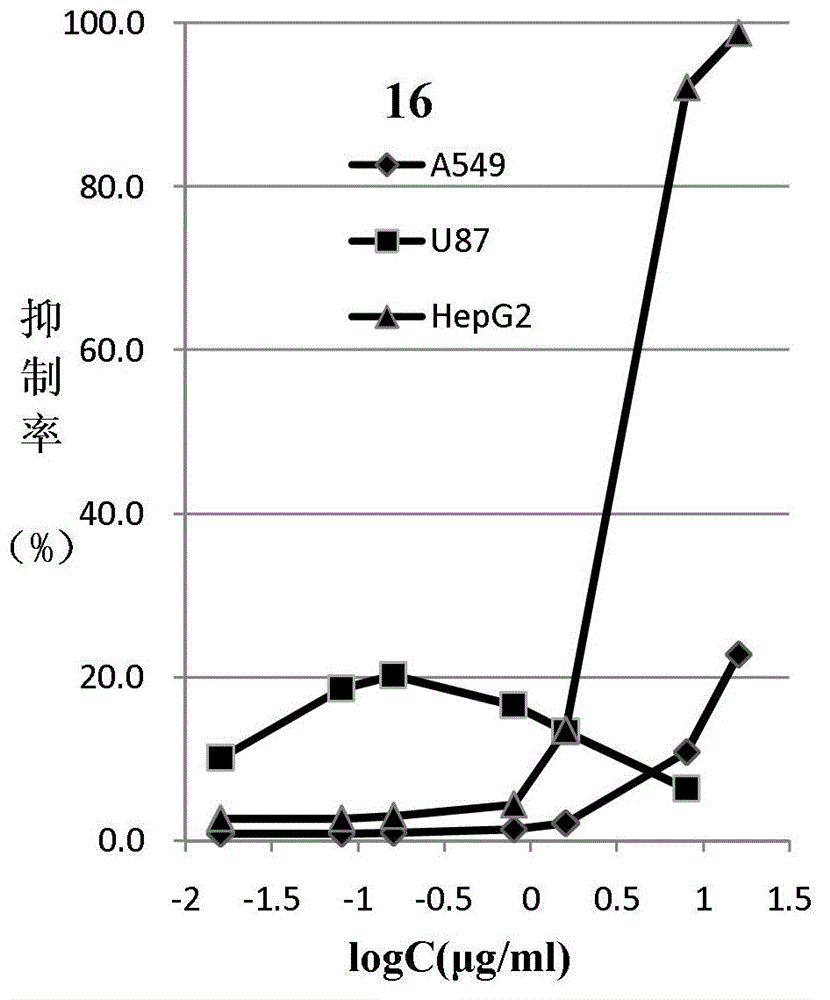
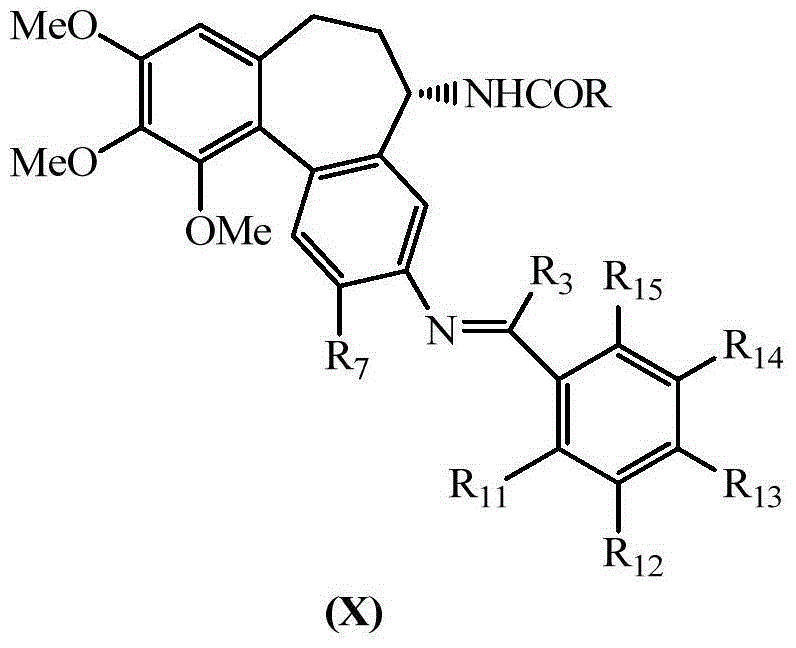
Colchicine derivative
A technology for colchicine and derivatives, applied in the field of colchicine derivatives, can solve problems such as weak effect, and achieve the effects of high cell selectivity and good anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] The synthesis of embodiment 1 compound 1
[0111]
[0112] Compound 1, which converts the C ring of colchicine into aniline, can be synthesized by the classic Fernholz method (see JustusLie bigs Ann. 1950), or it can be synthesized by referring to the method disclosed in the Chinese invention with application number 201210049085.3, and synthesized according to the above chemical reaction equation.
Embodiment 2
[0113] The synthesis of embodiment 2 compound 2
[0114]
[0115] 2.75g of compound 1 was dissolved in 65mL of 0.12M HCl, 13g of sodium sulfate was added, 2.5g of hydroxylamine hydrochloride and 2.1g of chloral were added successively under stirring, and the reaction was carried out at 55°C for 24 hours. 3 × 50mLEtOAc extraction (that is, extraction 3 times, each time with 50mLEtOAc), anhydrous Na2SO4 drying, silica gel column chromatography (EtOAc / EtOH=10:1, that is, the mobile phase composition is a mixture of EtOAc and EtOH with a volume ratio of 10:1 liquid), to obtain 3.3 g (yield 100%) of product 2 as a light yellow solid.
[0116] Compound 2H-NMR (500MHz, d 6 -DMSO): 1.90 (1H, s), 1.94 (1H, m), 2.0~2.2 (2H, m), 2.54 (1H, m), 3.49 (3H, s), 3.79 (3H, s), 3.84 ( 3H,s),4.49(1H,m),6.78(1H,s),7.28(1H,d),7.63(1H,dd),7.67(1H,d),7.72(1H,s),8.42(1H ,d), 10.24(1H,s), 12.19(1H,s). Compound 2 HRMS (high resolution mass spectrometry): C 22 h 25 N 3 o 6 Na + Theoretical va...
Embodiment 3
[0117] The synthesis of embodiment 3 compound 3 and compound 4
[0118]
[0119] 95mL of methanesulfonic acid was preheated to 40°C, 2.5g of compound 2 was added under stirring, the temperature was slowly raised to 85°C, and the reaction was carried out for 1 hour. After cooling, it was poured into 500mL ice water, extracted with 10×50mL EtOAc, washed with saturated brine, saturated aqueous sodium bicarbonate solution, saturated brine, and anhydrous Na 2 SO 4 Drying, silica gel column chromatography (CH 2 Cl 2 / MeOH=10:1), 1.0 g (yield 36%) of red solid product compound 3 and 0.3 g (yield 10%) of compound 4 were obtained.
[0120] Compound 3H-NMR (500MHz, d 6 -DMSO): 1.89 (3H, s), 1.96 (1H, m), 2.1~2.2 (2H, m), 2.56 (1H, m), 3.52, (3H, s), 3.79 (3H, s), 3.84 (3H, s), 4.47 (1H, m), 6.80 (1H, s), 6.89 (1H, s), 7.37 (1H, s), 8.56 (1H, d, J8Hz), 11.09 (1H, s).
[0121] Compound 3 HRMS: C 22 h 22 N 2 o 6 The theoretical value is 410.1478; the measured value is 410.1479...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com