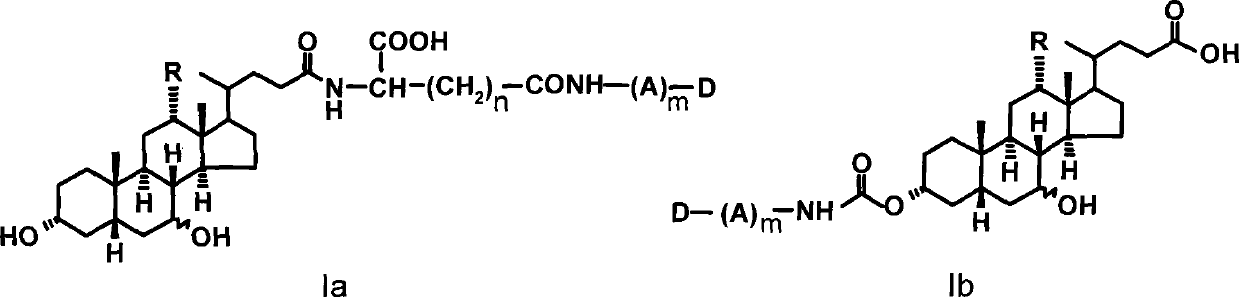Colchicine Derivative-Bile Acid Conjugate and Its Medical Application
The technology of colchicine and colchic acid is applied in the application field of preparing liver cirrhosis and liver fibrosis treatment drugs, and can solve the problems of complex chemical composition, small drug loading and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The present invention can be further described by the following examples, however, the scope of the present invention is not limited to the following examples. Those skilled in the art can understand that various changes and modifications can be made in the present invention without departing from the spirit and scope of the present invention. Example 1 Preparation of N-cholylglutamic acid-trimethylcolchicinic acid conjugate (Ia-1)
[0028] 1.1 Synthesis of N-choylglutamic acid-α-methyl ester
[0029] 16.1 g (0.1 mol) of glutamic acid-α-methyl ester was dissolved in 160 ml of 1N sodium hydroxide solution, and the mixture was cooled to 0°C. Take 36.7 grams (90 mmol) of cholic acid and dissolve it in 110 milliliters of tetrahydrofuran, and cool it to -15°C in an ice salt bath; add 5.20 milliliters of N-methylmorpholine and 6.02 milliliters of isobutyl chloroformate in turn, React in the bath for 8-10 minutes. The glutamic acid-α-methyl ester solution was added to the r...
Embodiment 2
[0034] Example 2 Preparation of N-ursodeoxycholoylglutamic acid-trimethylcolchicinic acid conjugate (Ia-2)
[0035] With ursodeoxycholic acid instead of cholic acid, refer to the method of Example 1 to obtain compound Ia-2, yield: 72%, TLC: chloroform: methanol = 20: 1, R f = 0.5. MS (m / e): 847.0 (M+1); elemental analysis (C 48 h 66 N 2 o 11): theoretical value C 68.06%, H 7.85%, N3.31%; experimental value C 68.12%, H 7.74%, N3.40%.
Embodiment 3
[0036] Preparation of Example 3N-cholylglutamic acid-deacetylcolchicine conjugate (Ia-3)
[0037] With deacetylcolchicine instead of trimethylcolchic acid, referring to the method of Example 1, compound Ia-3 was obtained, yield: 58%, TLC: chloroform: methanol = 25: 1, R f = 0.3. MS (m / e): 877.3 (M+1); elemental analysis (C 49 h 68 N 2 o 12 ): theoretical value C 67.10%, H 7.81%, N3.19%; experimental value C 67.25%, H 7.70%, N3.24%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com