Amiodarone hydro chloride dispensible tablet, and its preparing method
A technology of amiodarone hydrochloride and dispersible tablets, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc., and can solve the large impact of absorption in the body, the absorption of amiodarone hydrochloride tablets, and the difference in bioavailability And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
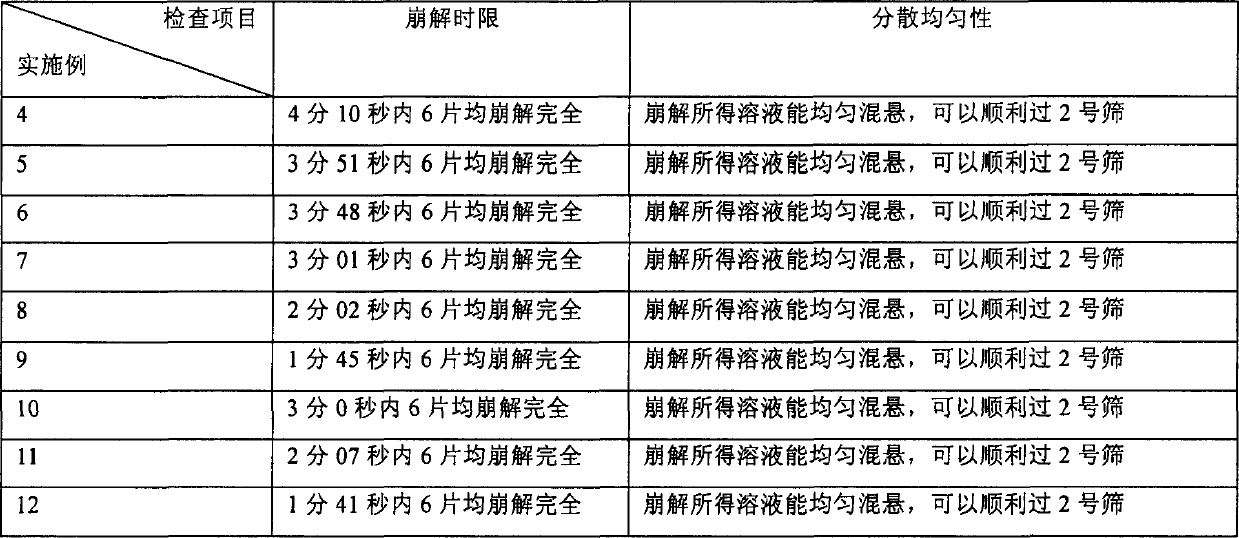
Embodiment 1
[0071] Get raw materials: Amiodarone hydrochloride 200g, microcrystalline cellulose 100g, sodium starch glycolate 60g, 4g Tween-80, povidone K30 solution (3%, w / v; 50% ethanol solution) 120ml, micropowder silica gel 10g , stevia 2g, magnesium stearate 5g, prepare according to the following steps:
[0072] Get amiodarone hydrochloride, microcrystalline cellulose and 30g carboxymethyl starch sodium, amiodarone hydrochloride is micronized, with 95% powder particle size being less than 10 μ m, average particle diameter (50% powder body particle diameter) is less than 5 μ m as micropowder Mix well; dissolve Tween-80 in an appropriate amount of povidone K30 solution, add it to the above mixture to make a soft material, and continuously rinse the container with povidone K30 solution, add the washing liquid to the soft material, Until the soft material meets the granulation requirements, pass through a 24-mesh sieve to granulate, and dry at 55°C by air blast; take the dried granules a...
Embodiment 2
[0074] Raw materials: 200g of amiodarone hydrochloride, 50g of microcrystalline cellulose, 13g of crospovidone XL-10, an appropriate amount of povidone K30 solution (3%, w / v; 50% ethanol solution), micronized silica gel 10g, stevia Su 2g, magnesium stearate 5g, prepare according to the following steps:
[0075] Weigh the raw material of micronized amiodarone hydrochloride, microcrystalline cellulose and half amount of crospovidone XL-10, and mix them evenly; Granulate with a 24-mesh sieve and blow dry at 55°C; take the dried granules and pass through a 20-mesh sieve for granulation, add the remaining amount of crospovidone XL-10 and the prescribed amount of magnesium stearate, and mix well; use φ= 8.5mm shallow concave punched tablet, made into 1000 pieces, the hardness of the tablet is controlled at 3-6kg, and the tablet weight is 280mg.
Embodiment 3
[0077] Get raw materials: amiodarone hydrochloride 200g, microcrystalline cellulose 100g, 30g crospovidone XL-10, 6.8g Tween-80, povidone K30 solution (3%, w / v; 50% ethanol solution) Appropriate amount, micropowder silica gel 10g, stevioside 2g, magnesium stearate 5g, prepare according to the following steps:
[0078] Weigh the micronized amiodarone hydrochloride raw material, microcrystalline cellulose and crospovidone XL-10, and mix them evenly; dissolve the prescription amount of Tween 80 in an appropriate amount of povidone K30 solution, and add it to the above mixture to prepare Soft materials, and continuously rinse the container with povidone K30 solution, add the washing solution to the soft materials until the soft materials meet the granulation requirements, pass through a 24-mesh sieve to granulate, and blow dry at 55 °C; take the dried granules and pass through Sieve through a 20-mesh sieve, add the remaining amount of crospovidone XL-10 and the prescribed amount o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet weight | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com