Thermostable lactase preparation method
A lactase, high temperature-resistant technology, applied in the biological field, can solve the problems of low expression and insufficient large-scale production, and achieve the effects of simple steps, low cost, and improved nutritional value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
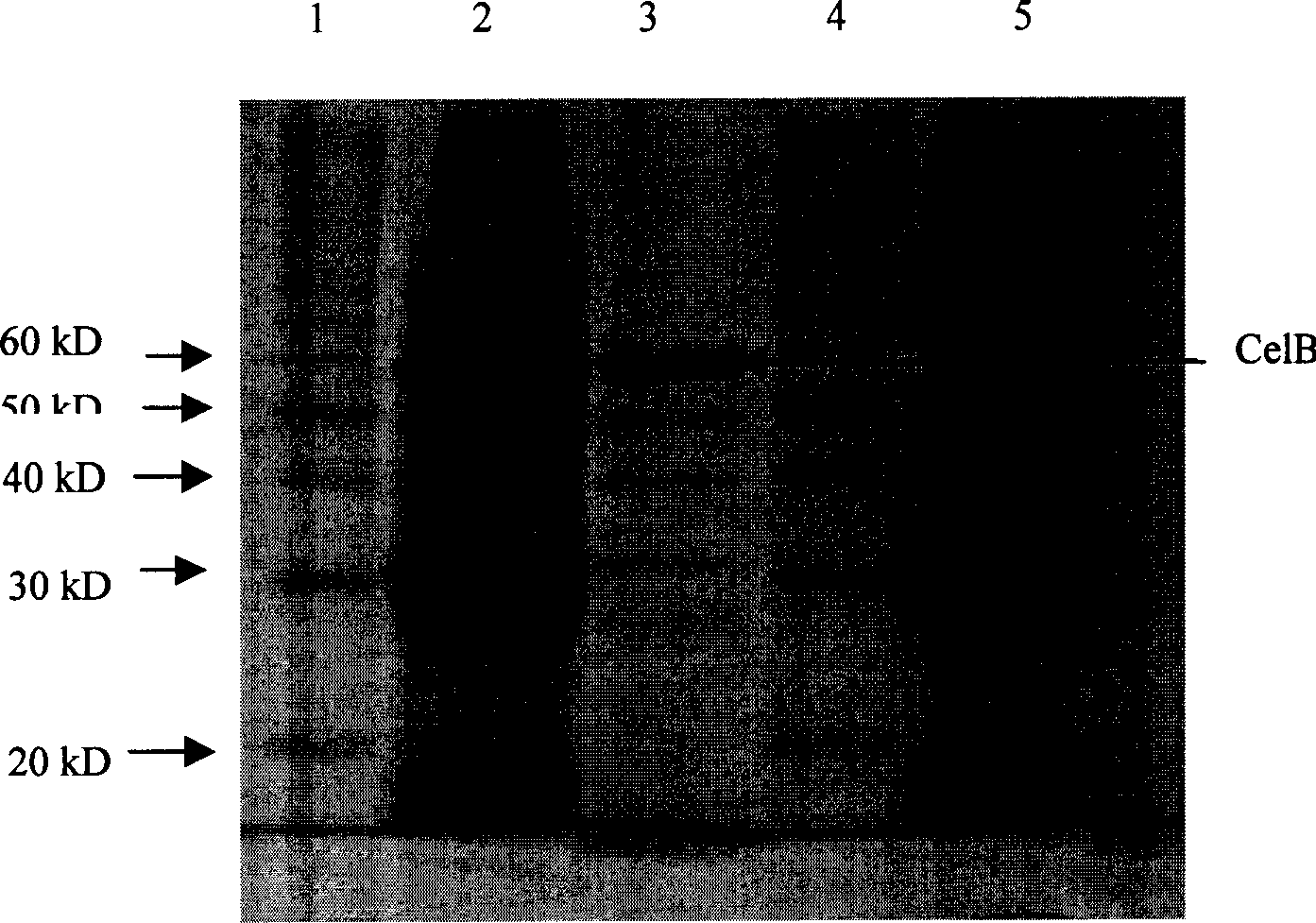
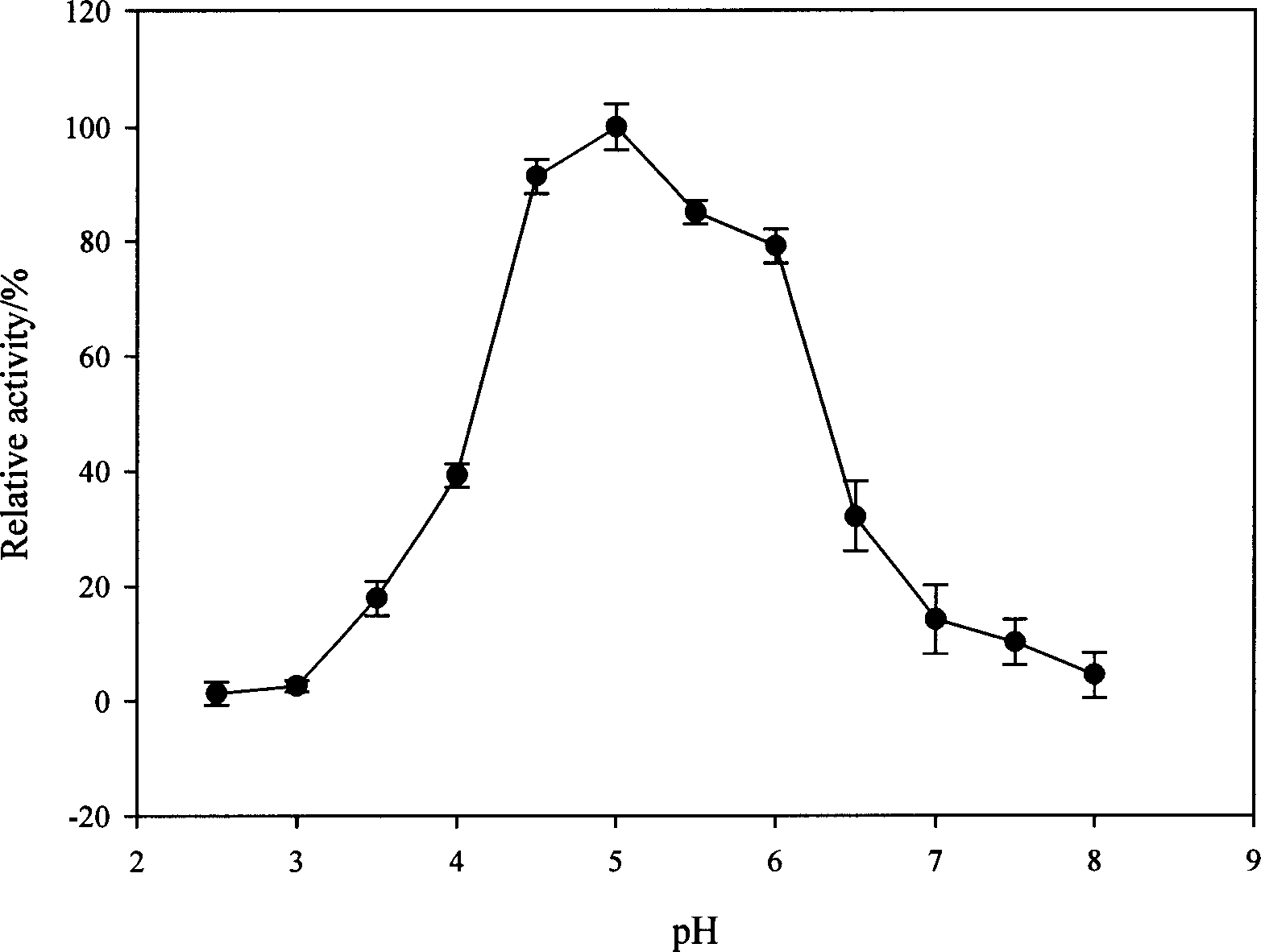
Image
Examples
Embodiment 1
[0034] Embodiment 1, cloning and sequence analysis of CelB gene
[0035] 1. Preparation of genomic DNA of Pyrococcus furiosus containing CelB gene
[0036] (1) Pick a single colony and culture overnight.
[0037] (2) Centrifuge at 12,000 rpm for 2 minutes, and remove the supernatant.
[0038] (3) Add 500 μl of 0.05M Tris-Cl (pH 8.0) buffer containing 25% sucrose to the precipitation, mix and resuspend, add 100 μl of 5 mg / ml lysozyme, mix well, and incubate at 20°C for 1 hr.
[0039] (4) Add SET solution (150 mM NaCl, 1 Mm EDTA, 20 mM Tris-Cl pH 8.0), 500 μl 5% SDS and 100 μl 20 mg / ml proteinase K, mix well, and incubate at 37° C. for 1 hr.
[0040] (5) Add an equal volume of chloroform / isoamyl alcohol (24:1), mix well, centrifuge at 11,000 g for 5 min, and transfer the supernatant to a new tube.
[0041] (6) Add an equal volume of phenol / chloroform / isoamyl alcohol (25:24:1), mix well, centrifuge at 11,000 g for 5 min, and transfer the supernatant to a new tube.
[0042] (7...
Embodiment 2
[0073] Embodiment two, the construction of recombinant transfer vector pVL94 (CelB)
[0074] Firstly, a pair of mutant primers were designed, the primer sequences are as follows:
[0075] Primer1: 5'gggatcccgatggtgggggtgtatgaataattcggaatattt
[0076] Primer2: 5'aaagcttaactttatcc aataatatat tatgtata
[0077] Primer1 mutates the start codon ATG of the polyhedron to ATT, and at the same time introduces a BamH I site at the 5' end, retaining the 35bp polyhedron gene sequence to stabilize the transcription product of the foreign gene and improve translation efficiency; in the reverse direction The HindIII site was introduced into Primer2 to facilitate gene cloning.
[0078] The DNA of the wild BmNPV was used as a template for PCR amplification, and the amplified fragment was double digested with BamH I and HindIII, and then cloned into the HindIII and BamH I sites of pUC18 (purchased from Gibco Company) to obtain the vector pUC-BH. The pVL1393 vector (purchased from Clontech) was ...
Embodiment 3
[0080] Embodiment three, the reproduction of Bombyx mori nuclear polyhedrosis virus parent strain BmNPV and the preparation of virus DNA
[0081] Prepare 1 × TC-100 medium according to the product instructions of GIBCO Company, adjust the pH to 6.22 with 2N NaOH, add 10% fetal bovine serum to the filter-sterilized medium, and cultivate silkworm cell Bm-5 at 27°C. Infect about 50ml of cells in the logarithmic growth phase with the parental strain of Bombyx mori nuclear polyhedrosis virus BmNPV, and the multiplicity of infection is 1. After 3 to 4 days, collect the virus infection solution, centrifuge (5,000rpm×10min), remove the precipitate, and centrifuge the supernatant at 25,000rpm 1 hour, remove the supernatant, suspend the virus particle pellet with 1ml virus DNA extraction solution (1,000ml containing 12.1g Tris, 33.6g EDTA, 14.1g KCl, pH7.5), transfer to a 1.5ml centrifuge tube, add protease K to a final concentration of 50 μg / ml, incubated at 50°C for 2 hours, then adde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com