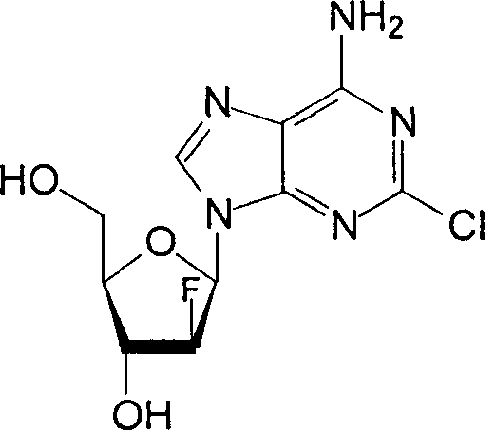
Injectable clofarabine composition
A technology of clofarabine and composition, which is applied in the field of pharmaceutical preparations, can solve problems such as difficulties, increased costs, and low solubility of clofarabine, and achieve the effect of good clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Clofarabine Sodium Chloride Injection [100ml contains clofarabine 20mg, that is, the concentration is 0.02% (g / ml)]
[0024] prescription:
[0025] Clofarabine 0.2g
[0026] Lactic acid 0.4ml
[0028] Water for injection up to 1000ml
[0029] Preparation process: Add 0.4ml of medicinal lactic acid to 100ml of water for injection, mix well, add clofarabine under stirring to dissolve; decolorize with activated carbon, filter to remove carbon; dilute to 850ml with water for injection.
[0030] Add sodium chloride to 100ml water for injection, heat to dissolve; decolorize with activated carbon, filter to remove carbon.
[0031] Slowly add the sodium chloride solution to the clofarabine dilution under stirring, adjust the pH value to 4 with 1→10 lactic acid, and add water for injection to the required amount. Filter with a 0.45μm filter membrane, fill in 100ml per bottle into an infusion bottle, and sterilize...
Embodiment 2
[0033] Clofarabine injection [2ml contains clofarabine 20mg, that is, the concentration is 1% (g / ml)]
[0034] prescription:
[0035] Clofarabine 10g
[0036] Citric acid 7g
[0037] Water for injection up to 1000ml
[0038] Preparation process: Add citric acid to 950ml of water for injection, stir to dissolve; add clofarabine, heat to 40°C, stir to dissolve, add water for injection to 1000ml (at this time, the pH of the solution is about 4.0). Filter and fill in 2ml ampoules. Melt and sterilize.
[0039] When the injection is used clinically, it needs to be diluted with 5% glucose injection or 0.9% sodium chloride injection and injected intravenously.
Embodiment 3
[0041] Clofarabine for injection (each bottle contains clofarabine 20mg)
[0042] prescription:
[0043] Clofarabine 20g
[0044] Lactic acid 8.0ml
[0045] Mannitol 80g
[0046] Water for injection up to 2000ml
[0047] Prepare 1000 bottles
[0048] Preparation process: Add 8.0ml of medicinal lactic acid to 1900ml of water for injection, mix well, add clofarabine, heat to 45°C, stir to dissolve, then add mannitol, stir to dissolve; add water for injection to 2000ml, adjust pH with lactic acid The value is at 4.5. Sterilize and filter, the filtrate is quantitatively filled in 7ml vials at a rate of 2ml / bottle, and is prepared into a freeze-dried powder through a freeze-drying process.
[0049] Reconstitution test At room temperature, add 1ml of water for injection, or 1ml of 0.9% sodium chloride injection, or 1ml of 5% glucose injection to 1 bottle of lyophilized powder, shake slightly, and dissolve into a clear solution in about 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com