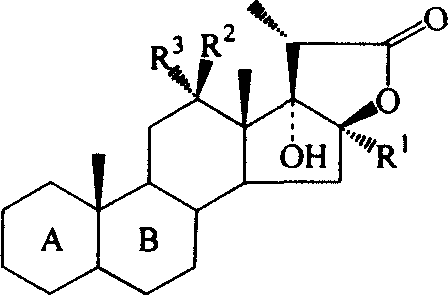
C17-hydroxy-steroid lactone antitumour medicine
An anti-tumor drug and anti-tumor technology, applied in the direction of anti-tumor drugs, drug combinations, steroids, etc., to achieve the effect of simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
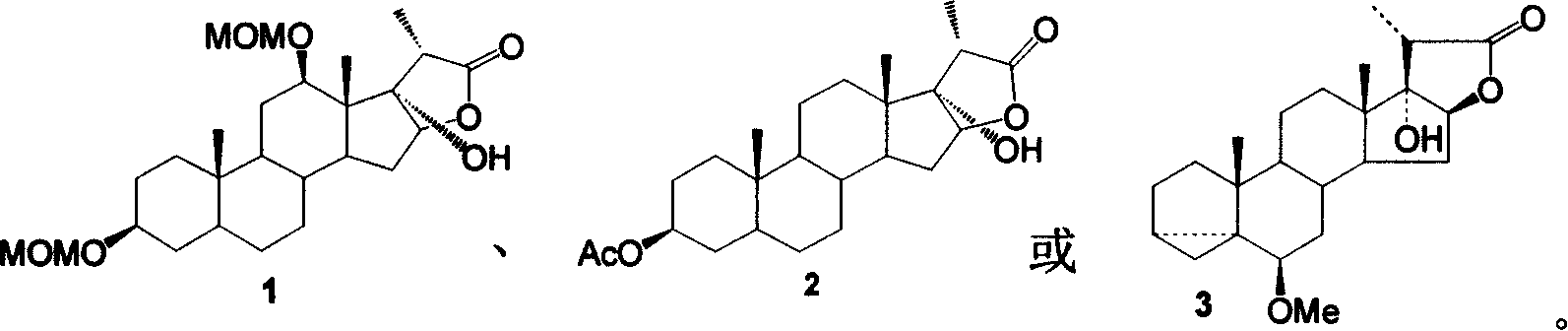
[0019] The synthesis of embodiment 1 compound 1
[0020]
[0021] Dissolve 50mg of compound 4 and 45mg (10eq) LiOH in a mixed solution of hydrogen peroxide (30%) and ethanol, react in an oil bath at 40°C until the raw materials disappear; neutralize dilute hydrochloric acid to partial acidity, and fully extract with ethyl acetate; use Washed with saturated brine, anhydrous Na 2 SO 4 Drying, concentration, and column chromatography yielded 45 mg of compound 1, Yield: 90%.
[0022] Compound 1 data:
[0023] C 26 h 42 o 7 466;
[0024] 1 HNMR (CD 3 COCD 3 ): 4.78 (1H, 12-OCH 2 OCH 3 , AB, J=5.9Hz), 4.69 (2H, 3-OCH 2 OCH 3 , s), 4.68 (1H, 12-OCH 2 OCH 3 , AB, J=5.9Hz), 4.58 (1H, 16-H, d-d, J 1 =7.8Hz,J 2 =5.0Hz), 3.937 (1H, 12-H, d-d, J 1 =10.8Hz,J 2 =5.0Hz), 3.55(1H, 3-H, m), 3.40(3H, 12-OCH 2 OCH 3 , s), 3.35 (3H, 3-OCH 2 OCH 3 , s), 2.88(1H, 20-H, q, J=7.8Hz), 2.36(1H, 15-H, m), 1.28(3H, 21-H, d, J=8.0Hz), 0.93(3H , 18-H, s), 0.87...
Embodiment 2
[0030] The synthesis of embodiment 2 compound 2
[0031]
[0032] Dissolve 50 mg (0.12 mmol) of compound 5 in 5 ml of acetone, add 0.05 ml of H 2 SO 4 , 20mg CrO 3 (0.2mmol) and 1ml of water were prepared as an oxidizing agent, then raised to room temperature and stirred for reaction, followed by TLC until the end of the reaction. The solid was removed by suction filtration, and the 2 Cl 2 Washed several times, the filtrate was washed with a large amount of water, saturated NaHCO 3 The solution was washed with saturated brine until neutral, dried over anhydrous magnesium sulfate, concentrated, and subjected to flash column chromatography to obtain 13 mg of compound 2, Yield: 26%.
[0033] Compound 2 data:
[0034] C 24 h 36 o 5 404;
[0035] 1 HNMR (CDCl3): 4.70 (m, 1H, 3-H), 4.40 (m, 1H, 16-H), 2.40 (m, 1H, 20-H), 2.00 (s, 3H, CH 3 CO), 1.40(d, J=8.0Hz, 3H, 21-H), 1.30(3H, s, 18-H), 0.75(3H, 19-H, s);
[0036] IR(γmax, cm -1 ): 3512, 2...
Embodiment 3
[0042] The synthesis of embodiment 3 compound 3
[0043]
[0044] In a 50 mL three-necked flask, add 628 mg of epoxy acid 6 (1.68 mmol) and 15 mL of absolute ethanol, and stir to dissolve. Add 710mg LiOH·H 2 O (16.8mmol), add 5mL of 30% hydrogen peroxide dropwise, stir and gradually raise the temperature to 80°C and react for ~24h (additional H 2 o 2 2mL), TLC tracking (2:1 n-C 6 h 14 -AcOEt expansion). After the reaction was completed (at this moment, what was generated was an intermediate product—diol), and saturated NaHSO 3 Remove excess hydrogen peroxide, add saturated NH 4 The aqueous Cl solution was neutralized to acidity, the organic phase was separated, and the aqueous phase was extracted once with 100 mL of ethyl acetate. The organic phase was washed twice with water and saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, and the solvent was recovered under reduced pressure, and the obtained oily crude...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com