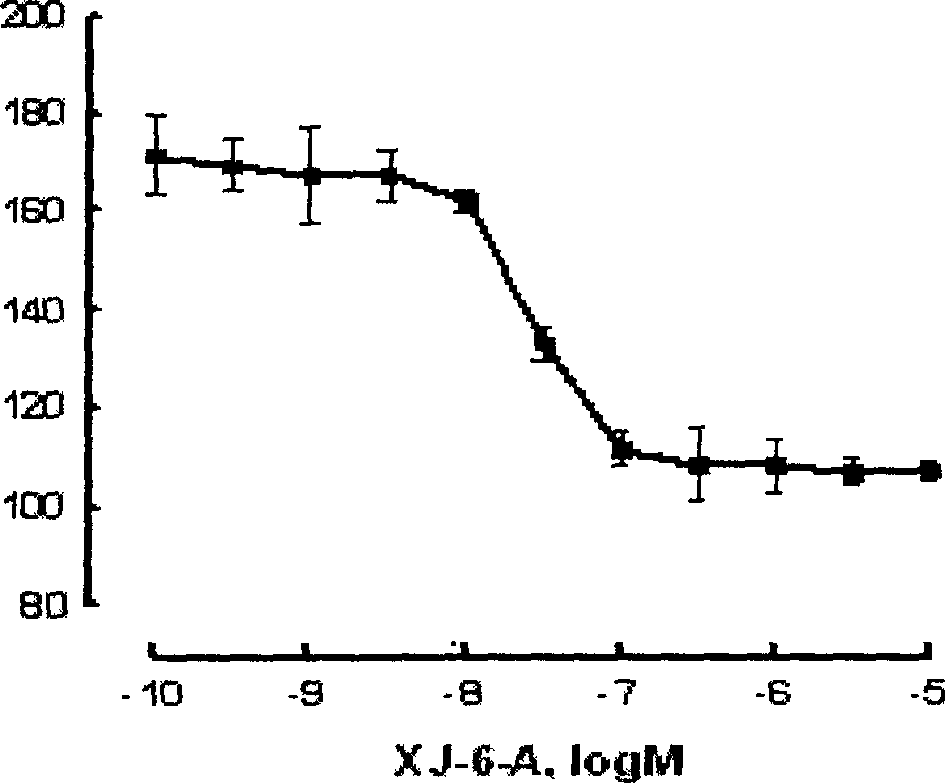
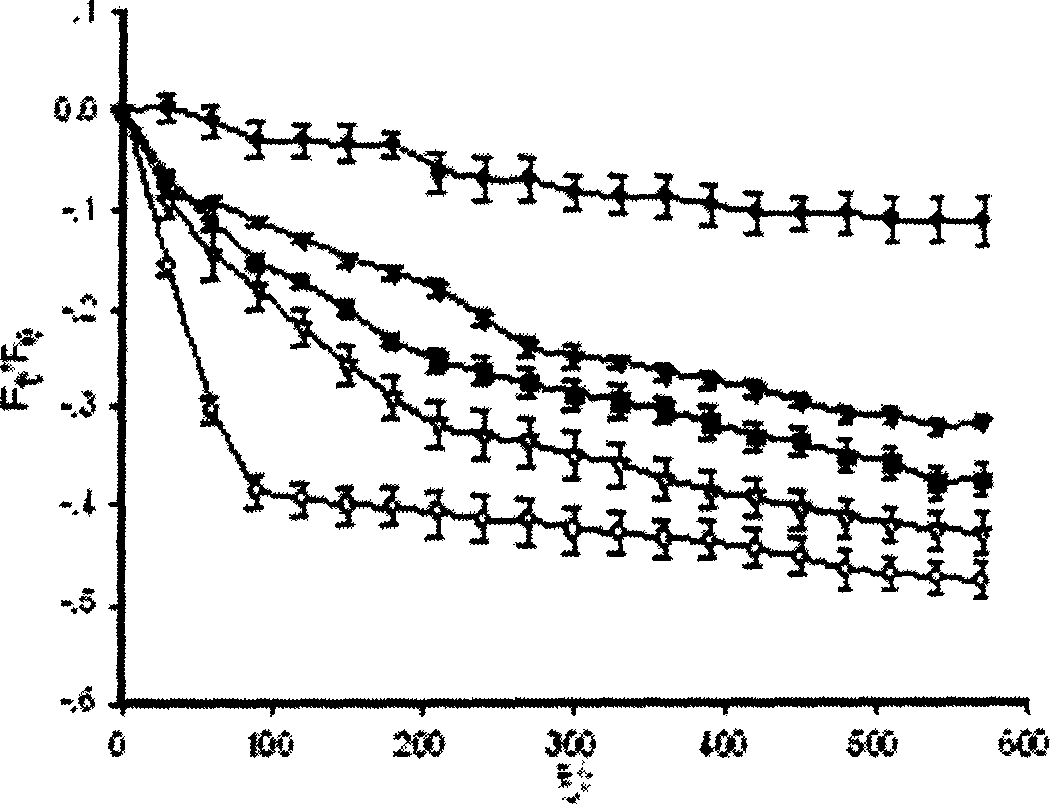
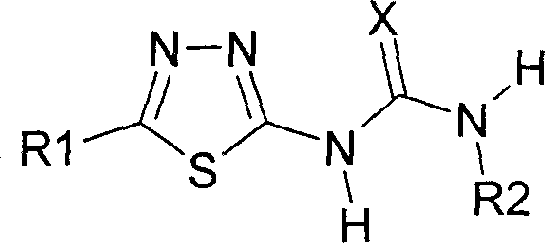
Protein antagonist with water channel
An aquaporin and antagonist technology, which is applied in the field of aquaporin antagonists to achieve the effects of high accuracy, less interference and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 N 1 -(5-n-Butyl-1,3,4-thiadiazol-2-yl)-N3-m-chlorophenylurea
[0023] A. Synthesis of intermediate 5-n-butyl-2-amino-1,3,4-thiadiazole
[0024] Put 0.05mol of n-valeric acid and 0.07mol of thiosemicarbazide into a 100mL three-necked flask respectively, add 11mL of concentrated sulfuric acid dropwise under stirring at room temperature, then heat for 8h, keep the temperature at 80-90°C, cool to room temperature, and react Slowly pour the solution into ice water, neutralize it with 40% sodium hydroxide solution to pH=8-9, let it sit overnight, filter it with suction, wash the solid crude product with water for 2-3 times, and recrystallize it with water or dilute ethanol solution to obtain Pure product, yield 70%, m.p.219-220°C.
[0025] B.N. 1 -(5-n-butyl-1,3,4-thiadiazol-2-yl)-N 3 -Synthesis of m-chlorophenylurea
[0026] Dissolve 2mmol of 5-n-butyl-2-amino-1,3,4-thiadiazole in 10mL of anhydrous acetonitrile, slowly add 2mmol of m-chlorophenyl isocyanate in...
Embodiment 25
[0030] Example 25 N 1 -(5-n-butyl-1,3,4-thiadiazol-2-yl)-N 3 -3,4-Dichlorophenylthiourea
[0031] Dissolve 2mmol of 5-n-butyl-2-amino-1,3,4-thiadiazole in 10mL of anhydrous acetonitrile, slowly add 2mmol of 3,4-dichlorophenylisothiocyanate dropwise at room temperature After adding 2 mL of anhydrous acetonitrile solution, reflux until the reaction was complete (TLC followed the reaction progress). After cooling, a large amount of white solids were precipitated, and the precipitated solids were filtered, followed by column chromatography with ethyl acetate / petroleum ether to obtain a pure product with a yield of 70%, m.p.>260°C.
[0032] 1 H-NMR (300MHz, DMSO-d 6 ): δ0.89(t, 3H, CH 3 ), 1.34(m, 2H, CH2), 1.65(m, 2H, CH2), 2.84(t, 2H, CH2), 7.42~7.69(m, 3H, C6H3), 10.05(s, 1H, NH), 13.78 (bs, 1H, NH).
[0033] Example
Embodiment 40
[0034] Example 40 N 1 -(5-sulfonamido-1,3,4-thiadiazol-2-yl)-N 3 -O-Chlorophenylurea
[0035] A. Synthesis of intermediate 2-amino-5-sulfonamido-1,3,4-thiadiazole
[0036] Dissolve 5.0 g of acetazolamide in 60 ml of ethanol, add 6 ml of 12N hydrochloric acid solution, and heat to reflux for about 6 hours (TLC to follow up the reaction process). Ethanol was removed under reduced pressure, and a solid precipitated out. The solid was added dropwise with 1N sodium hydroxide solution in an ice-water bath until the pH was 8. The crude product was obtained by suction filtration, and the pure product was obtained by recrystallization with water. m.p=219~220°C, the yield is 75%.
[0037] B.N. 1-(5-sulfonamido-1,3,4-thiadiazol-2-yl)-N 3 -O-Chlorophenylurea
[0038] Dissolve 0.36g (2mmol) of 2-amino-5-sulfonamido-1,3,4-thiadiazole 1 in 12ml of acetonitrile, slowly add 2mmol of o-chlorophenyl isocyanate dropwise, and heat to reflux until the reaction Complete (TLC followed the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com