Finely prepared sustained-releasing formulation for coronary disease and preparation process thereof
A technique for slow-release preparations of coronary heart, which is applied in the field of refined coronary heart sustained-release preparations and its preparation, and can solve the problems of large dosage of refined coronary heart tablets and backward process conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
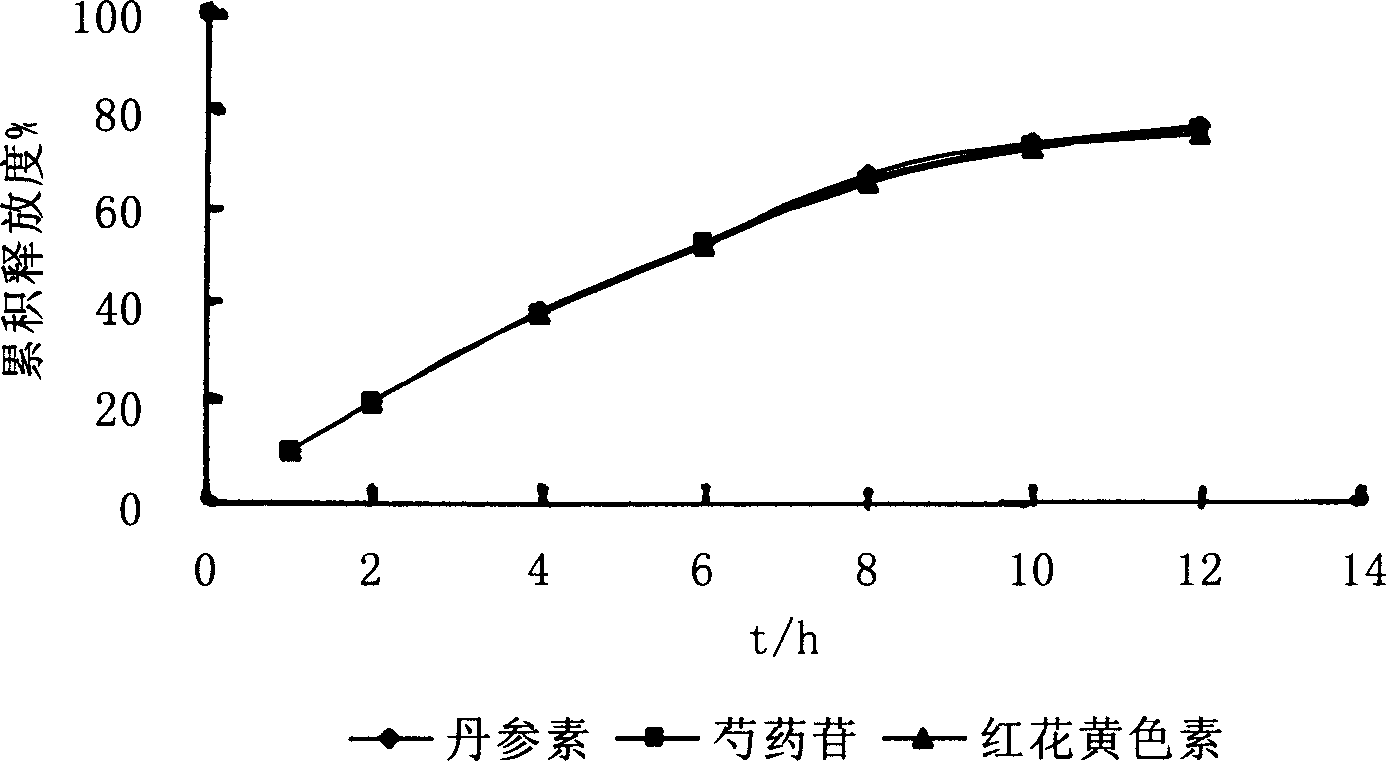
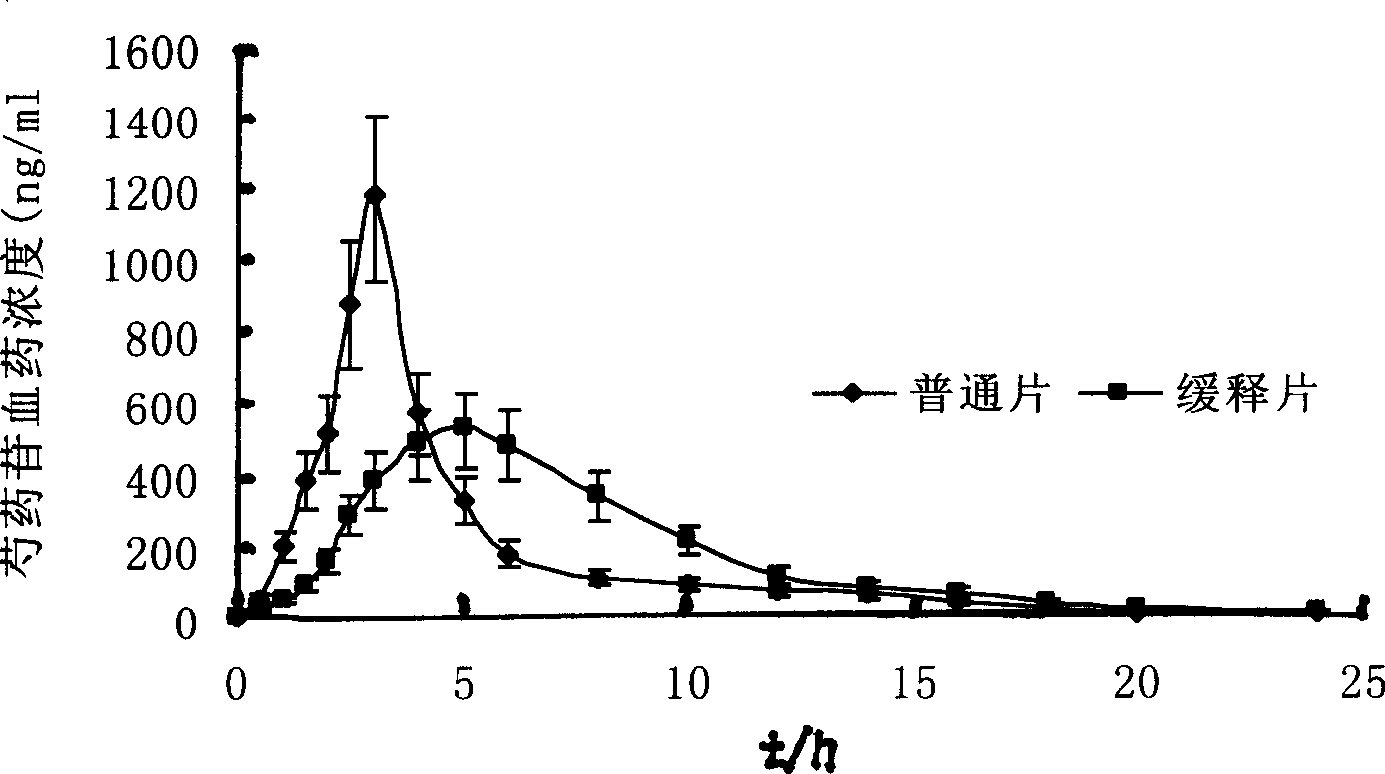
[0027] Extract and separate the various medicinal materials in the refined Guanxin formula, for example: extract the effective fraction group mainly composed of Danshensu by water extraction and alcohol precipitation method and / or other methods; use water extraction and alcohol precipitation method and / or 50%-98% After extraction with concentrated ethanol (including water), use macroporous resin separation method and / or other methods to extract the active fraction group mainly based on paeoniflorin; Porous resin separation method and / or other methods to extract the effective fraction group mainly composed of ferulic acid; after extraction with water extraction and alcohol precipitation method and / or different concentrations of ethanol (including water), use macroporous resin separation method and / or other methods Extract the active part group mainly composed of safflower yellow pigment; extract the volatile oil of balsamic balm by steam distillation and / or other methods, and in...
Embodiment 2
[0040] Each medicinal material in the refined Guanxin formula is extracted and separated with reference to Example 1, and the tablet of Example 2 made by a method known in the pharmaceutical industry contains the following ingredients by weight percentage:
[0041] Salvia Extract 23.0%
[0042] Paeoniae Rubra Extract 5.4%
[0043] Chuanxiong Extract 1.2%
[0044] Safflower Extract 11.5%
[0045] Aroma essential oil-β-cyclodextrin inclusion complex 7.9%
[0046] Bergamot Extract 0.7%
[0047] Carbopol 32.9%
[0048] Lactose 16.4%
[0049] Magnesium Stearate 1.0%
[0050] 60% ethanol solution appropriate amount
Embodiment 3
[0052] Each medicinal material in the refined Guanxin formula is extracted and separated with reference to Example 1, and the present Example 3 tablet made by a method known in the pharmaceutical industry contains the following ingredients by weight percentage:
[0053] Salvia Extract 27.5%
[0054] Radix Paeoniae Alba Extract 6.5%
[0055] Chuanxiong Extract 1.5%
[0056] Safflower Extract 13.8%
[0057] Aroma essential oil-β-cyclodextrin inclusion complex 9.5%
[0058] Bergamot Extract 0.8%
[0059] Sodium Alginate 35.5%
[0060] Calcium Alginate 3.9%
[0061] Magnesium Stearate 1.0%
[0062] 75% ethanol solution appropriate amount
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com