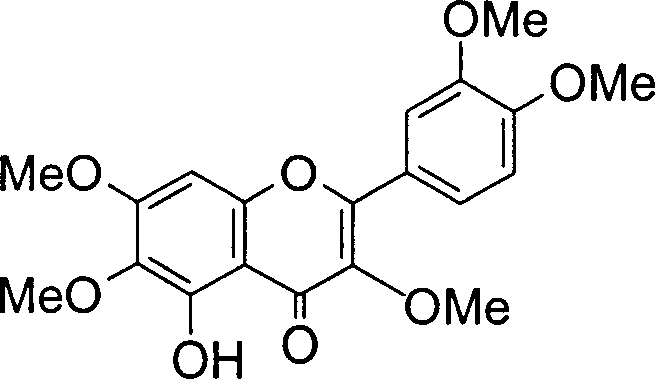
Laggera pterodonta buccal tablet, its preparation process and quality control method
A kind of panacea, appropriate amount of technology, applied in the field of traditional Chinese medicine preparation and its preparation method and the quality control of the preparation, can solve the problems of insufficient quality control, etc., and achieve the effect of preventing influenza, definite curative effect, and good antiviral effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A Lingdancao buccal tablet is mainly composed of traditional Chinese medicine Lingdancao, and the added accessories are a combination of various substances such as non-dairy creamer, aspartame, menthol, magnesium stearate, etc.; Coating, the coating material is a film coating premix.
[0028] The combination of Lingdancao dry ointment powder and auxiliary materials and the proportion by weight are: 30 parts of Lingdancao dry ointment powder, 28 parts of non-dairy cream, 3 parts of aspartame, 2.5 parts of menthol, magnesium stearate 1.5 parts, 0.5 part of volatile oil.
[0029] The lozenge preparation process is as follows:
[0030] Take 5000g of Lingdancao, steam distillation, collect its volatile oil, filter the distilled aqueous solution, and use the filtrate for use; add water to decoct the dregs for two times, 1.5-2 hours each time, filter, combine the three filtrates, and evaporate below 80°C Concentrate to thick paste, about 30% water or semi-solid after cooling...
Embodiment 2
[0032] A Lingdancao buccal tablet is mainly composed of traditional Chinese medicine Lingdancao, and the added accessories are: non-dairy creamer, aspartame, menthol, magnesium stearate and other combinations of substances, compressed into tablets and then packed Film coating, the coating material is a film coating premix.
[0033] The combination of Lingdancao dry ointment powder and auxiliary materials and the proportion by weight are: 30 parts of Lingdancao dry ointment powder, 28 parts of non-dairy cream, 3 parts of aspartame, 2.5 parts of menthol, magnesium stearate 1.5 parts, volatile oil 1.0 parts.
[0034] The lozenge preparation process is as follows:
[0035] Take 5000g of Lingdancao, steam distillation, collect its volatile oil, filter the aqueous solution after distillation, and use the filtrate for use; the volatile oil is included with β-cyclodextrin, the specific method is: take an appropriate amount of β-cyclodextrin 120g, add distilled water to prepare into ...
Embodiment 3
[0037] A Lingdancao buccal tablet is mainly composed of traditional Chinese medicine Lingdancao, and the added auxiliary materials are a combination of sucrose, lactose, xylitol, stevioside, menthol and magnesium stearate. Film coating is applied, and the coating material is a film coating premix.
[0038]The combination of Lingdancao dry ointment powder and auxiliary materials and the proportion by weight are: 30 parts of Lingdancao dry ointment powder, 10 parts of xylitol, 10 parts of sucrose, 10 parts of lactose, 5 parts of sodium cyclamate, and 2.5 parts of menthol parts, magnesium stearate 1.5 parts, volatile oil 2.0 parts.
[0039] The lozenge preparation process is as follows:
[0040] Take 5000g of Lingdancao, steam distillation, collect its volatile oil, filter the distilled aqueous solution, and use the filtrate for use; add water to decoct the dregs for two times, 1.5-2 hours each time, filter, combine the three filtrates, and evaporate below 80°C Concentrate to t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com