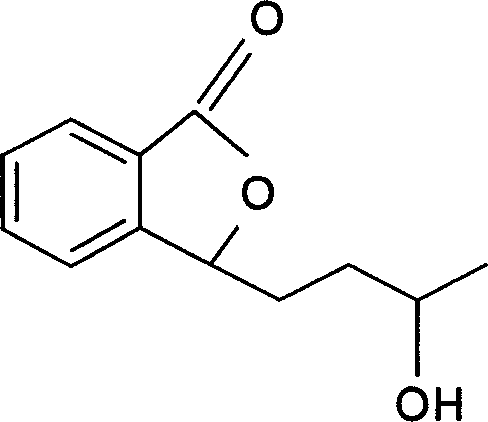
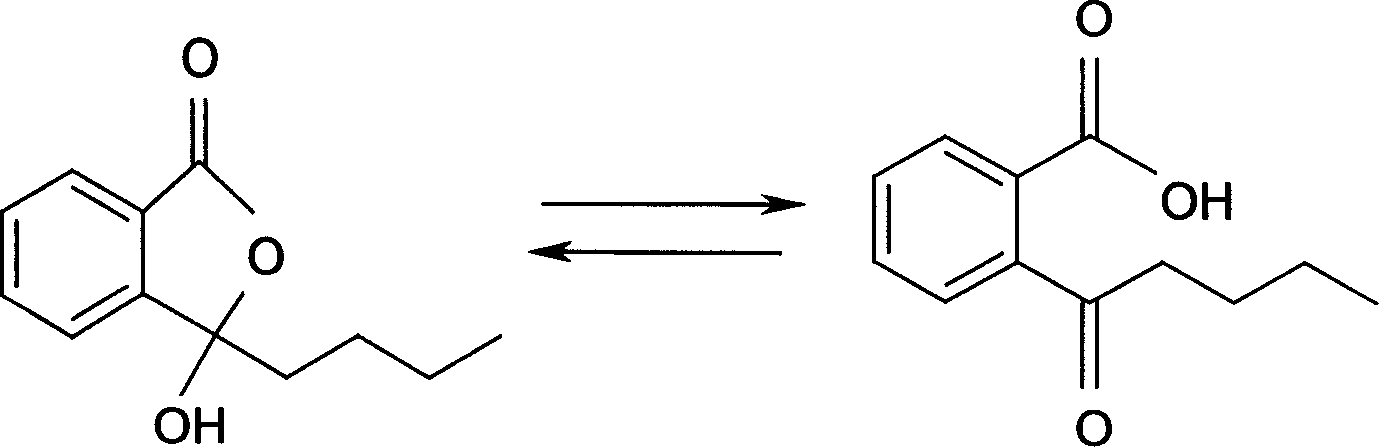
Application of butylbenzene phthalein homolog in preparation of medicine for treating cerebral ischemia disease
A technology of butylphthalide and cerebral ischemia, applied in blood diseases, nervous system diseases, cardiovascular system diseases, etc., can solve problems such as unclear pharmacodynamic effects, and achieve the effect of improving neurological symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, MI and MII are on the influence of neurological symptoms caused by traumatic brain injury in rats
[0032] Experimental animals: male Wistar rats
[0033] Experimental method: use a conical metal object weighing 220g to drop freely from a height of 30cm, and hit the rat's left coronal suture posterior nuchal bone, causing traumatic brain injury in the rat. After 5 minutes, oral administration: MI and MII (20, 40, 80mg / kg), 24 hours later, scoring and evaluating behavioral changes.
[0034] Experimental results: rats with neurological symptoms caused by traumatic brain injury accompanied by cerebral ischemia, after oral administration, compared with the solvent control group, the MI group can significantly improve the neurological symptoms in a dose-dependent manner. Compared with the control group, P<0.05, indicating that the two metabolites MI and MII of butylphthalide can improve the neurological symptoms caused by cerebral ischemia caused by traumatic ...
Embodiment 2
[0035] Embodiment 2, the impact of MI and MII on memory impairment caused by focal cerebral ischemia
[0036] Experimental instrument: shuttle box, others are the same as in Example 1
[0037] Experimental animal is the same as embodiment 1
[0038] experimental method:
[0039] (1) Carry out learning and memory acquisition training to rats:
[0040] (2) Cerebral artery ligation: According to the Tamura method, rats were subjected to cerebral artery ligation. After 24 hours, the experiment of measuring the learning and memory of the rats was repeated, and the method was the same as (1).
[0041] (3) Grouping and administration: divided into 9 groups, normal group, sham operation group, ischemia control group, MI and MII (15, 30, 60 mg / kg) groups. Oral administration was performed 15 minutes after the operation, and the changes in the latent period of the active and passive avoidance reactions of the drug on the rats were observed 24 hours later.
[0042] Experimental resul...
Embodiment 3
[0043] The effect of embodiment 3, MI and MII on rat cerebral artery ligation cerebral edema
[0044] Experimental animal is the same as embodiment 1
[0045] Experimental method: The right middle cerebral artery was ligated to the rats to cause cerebral edema. After 15 minutes, MI and MII (20, 40, 80 mg / kg) were administered orally, and they were killed in 24 hours. Ww. After baking at 100°C for 24 hours, the dry weight was measured, and the water weight of the brain tissue was calculated by the dry-wet method. The tissue was nitrated for 4 hours, the pH value was adjusted with HCI, and the ion-selective electrode was connected to the oxygen tissue analyzer to measure the Na in the brain tissue + 、K + concentration.
[0046] Experimental results: MI group reduced brain water volume and Na + content, the increase of K+ content is very significant, and there is a dose-effect relationship; the MII middle and high dose group has a more obvious effect, compared with the blank...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com