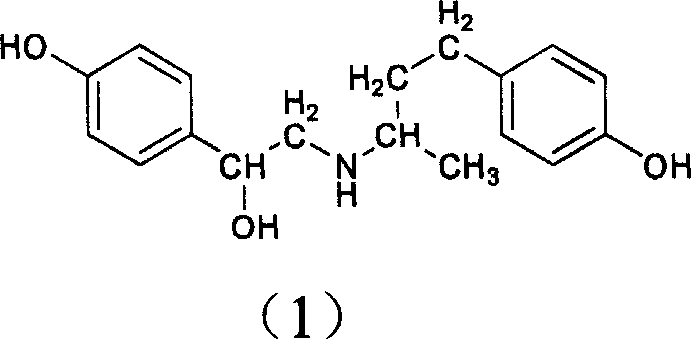
Method for preparing compound of ractopamine
A ractopamine and compound technology, which is applied in the field of compound preparation, can solve the problems of slow reaction rate, increased reaction time, and difficulty in completing the reaction, and achieves the effects of easy operation, simple steps and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Add 100g (0.61mol) of raspberry copper and 280g (2.95mol) of formamide into a 1000ml flask, mix roughly, and then add 140ml of acetic acid. React at 130°C for two hours, distill off a large amount of water, then raise the temperature to 150°C, and react for two hours. After cooling, 600ml of water was added, and extracted four times with 700ml of ethyl acetate. The ethyl acetate layer was extracted twice with 300 ml of water. Evaporate to dryness under reduced pressure to obtain a brown viscous liquid. Add 260ml of concentrated hydrochloric acid, reflux for 25min, the solution is tea green. After cooling, a large amount of white precipitate was produced, which was washed three times with 350 ml of ethyl acetate. After drying, a white powder was obtained, which was 1-methyl-3-(4-hydroxyphenyl)-propylamine hydrochloride, and the yield was 90%.
[0027] (2) Add 6.4g (0.032mol) 1-methyl-3-(4-hydroxyphenyl)-propylamine hydrochloride, 5.4g (0.025mol) ω-bromo-p-hydroxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com