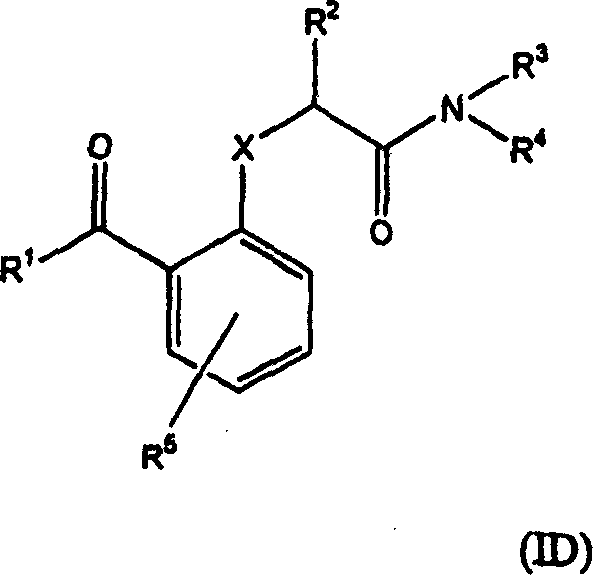
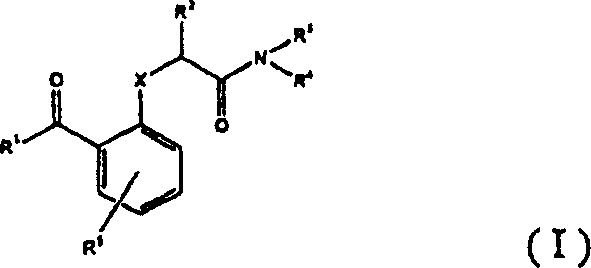
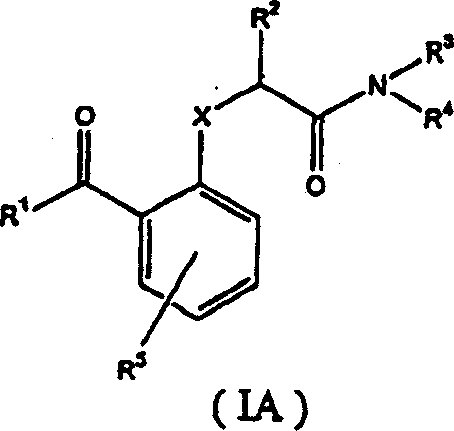
Benzophenones as inhibitors of reverse transcriptase
An alkyl and heterocycle technology, applied in the field of benzophenone as a reverse transcriptase inhibitor, can solve the problem of inactivity of drug-resistant strains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0317] The following is a flow chart showing the preparation of the compound of formula VI, where R 1 And R 5 As defined above, R 7 It is hydrogen or methyl. Where R 1 And R 5 As defined above and R 7 Compounds of formula VI that are methyl can be obtained by making R 5 As defined above and R 7 The compound of formula IX which is methyl and wherein R 1 And R 10 The compound of formula X as defined above is prepared by reaction, but with the further condition that these groups are chemically compatible with the reaction conditions, R 7 Is methyl, R 9 Is halogen, preferably bromine or iodine, and R 10 It is N, O-dimethylhydroxyamino.
[0318]
[0319] The compound of formula IX is usually treated with a reagent capable of carrying out a halogen-metal exchange reaction, such as sec-butyl lithium, methyl lithium, tert-butyl lithium, or more preferably n-butyl lithium. The halogen-metal exchange can be performed in an ether solvent, such as THF, dioxane or more preferably diethyl eth...
Embodiment 1
[0488]
[0489] Step A:
[0490]
[0491] The 2-bromo-4-chloroanisole (8.98g, 40.54mmol) in diethyl ether (65ml) was cooled to -78°C, and n-butyllithium (26ml of 1.6M solution in hexane was added via syringe, 41.6mmol). The resulting orange solution was stirred at -78°C for 30 minutes, after which only 2-thiazolecarboxaldehyde (4.53 g, 40.04 mmol) was added to form a reddish purple solution. The mixture was stirred at -78°C for 15 minutes, after which time water (50 ml) was added and the mixture was allowed to warm to room temperature. The mixture was poured into a separatory funnel containing ether and water. The organic layer was collected, washed with water, brine, dried over magnesium sulfate, filtered and the solvent was removed under reduced pressure to obtain a white solid. The solid was washed with hexane and dried under vacuum to obtain white needles (5.21 g, 51%). 1 H NMR(CDCl 3 , 400 MHz) δ 7.70 (d, J = 4 Hz, 1H), 7.38 (d, J = 4 Hz, 1H), 7.28 (d, J = 4 Hz, 1H), 7.23...
Embodiment 2
[0501]
[0502] Step A:
[0503]
[0504] According to general method II, phenol 4 (2.31 g, 9.64 mmol), potassium carbonate (6.95 g, 50.3 mmol), ethyl bromoacetate (1.1 ml, 1.7 g, 9.9 mmol) and acetone (150 ml) were used. The product was used in the next reaction without any further purification. 1 H NMR(CDCl 3 , 300MHz) δ 8.05 (d, J = 3Hz, 1H), 7.76 (d, J = 3Hz, 1H), 7.66 (d, J = 3Hz, 1H), 7.48 (dd, J = 9, 3Hz, 1H) , 6.93 (d, J = 9 Hz, 1H), 4.61 (s, 2H), 4.21 (q, J = 6 Hz, 2H), 1.26 (t, J = 6 Hz, 3H).
[0505] Step B:
[0506]
[0507] According to general method III, ester 6 (3.1 g, 9.6 mmol), THF (30 ml), water (10 ml), ethanol (10 ml) and lithium hydroxide (1.0 g, 23.8 mmol) were used. The product was used in the next reaction without any further purification. 1 H NMR(DMSO-d 6, 300 MHz) δ 8.30 (d, J = 3Hz, 1H), 8.15 (d, J = 3Hz, 1H), 7.63 (d, J = 3Hz, 1H), 7.57 (dd, J = 9, 3Hz, 1H) ), 7.05 (d, J=9 Hz, 1H), 4.45 (s, 2H).
[0508] Step C:
[0509] According to general meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com