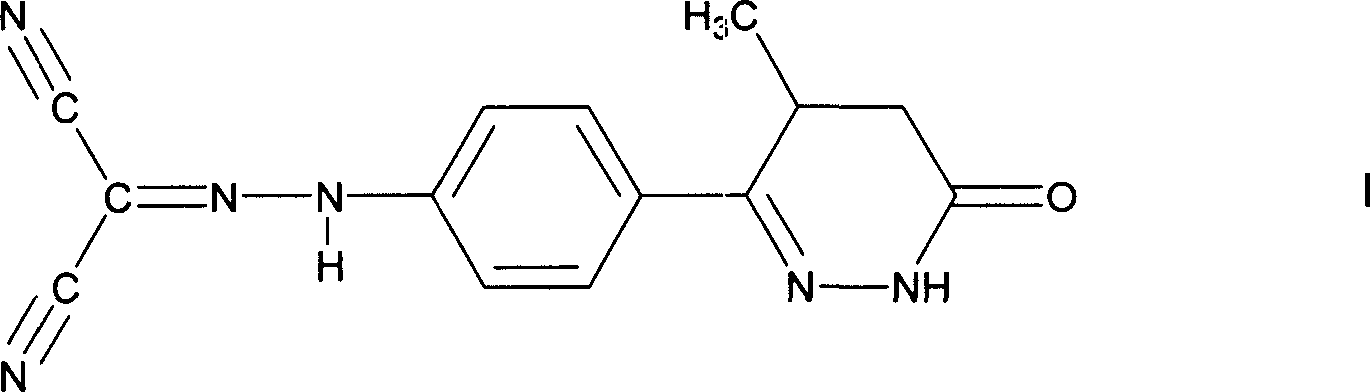
A combination treatment for acute myocardial infarction
A technology for acute myocardial infarction and mortality, applied in drug combinations, pharmaceutical formulations, cardiovascular system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1. Intravenous infusion of concentrate
[0043] (a) Levosimone 2.5mg / ml
[0044] (b) Kollidon PF12 10mg / ml
[0045] (c) Citric acid 2mg / ml
[0046] (d) Dehydrated ethanol about 1ml (785mg)
[0047] Preparation of concentrated solution: Add citric acid, Kollidon PF 121 and levosimendan into a sterile preparation container filled with dehydrated ethanol under stirring, and then filter the resulting solution with a 0.22mm sterile filter, and the filtrate is placed in a sterile Distribute into 8ml and 10ml (with 5ml and 10ml transfer) bottles between operations, and seal them with rubber stoppers.
[0048] This concentrated solution needs to be diluted with an aqueous vehicle before intravenous infusion, and is usually diluted with an aqueous isotonic vehicle (such as 5% dextrose aqueous solution or 0.9% sodium chloride aqueous solution) to obtain an aqueous intravenous solution, wherein the amount of levosimendan is usually about Within the range of 0.001-1.0 m...
Embodiment 2
[0049] Example 2. t-PA composition in lyophilized state
[0050] (a)t-PA 2.0% (w / w)
[0051] (b) Phosphoric acid 20% (w / w)
[0052] (c) L-Arginine 78% (w / w)
[0053] The above components are mixed by standard methods, lyophilized and sterilized, including 20 mg, 50 mg, or 100 mg t-PA / dose form (vial) of the lyophilized product reconstituted with sterile water for injection, e.g., to make 1 mg / ml of injection.
example 3
[0054] Example 3: Oral administration of levosimendan composition
[0055] Size 3 Hard Gelatin Capsules
[0056] Levosimone 2.0mg
[0057] Lactose 198mg
[0058] The pharmaceutical preparation in capsule form can be prepared by filling the powder after mixing levosimendan and lactose into capsules made of No. 3 hard gelatin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com