Treatment of glioblastoma with thymosin-alpha 1
A glioblastoma, thymosin technology, applied in antitumor drugs, peptide/protein components, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Treatment of 9L and 293 Tumor Cell Lines with Thymosin α1
[0028] Tumor cell line: Add 5% FCS, 2mM L-glutamine, and 100μM non-essential amino acid (Gibco-BRL, Grand Island, NY) Dulbecco's modified Eagle's medium (DMEM) to rat 9L glioblastoma Tumor cells and 293 human kidney cells were cultured. All cell lines were placed in 5% CO 2 in a humid atmosphere of 37°C. All cell lines were purchased from the American Type Culture Center and certified free of pathogens.
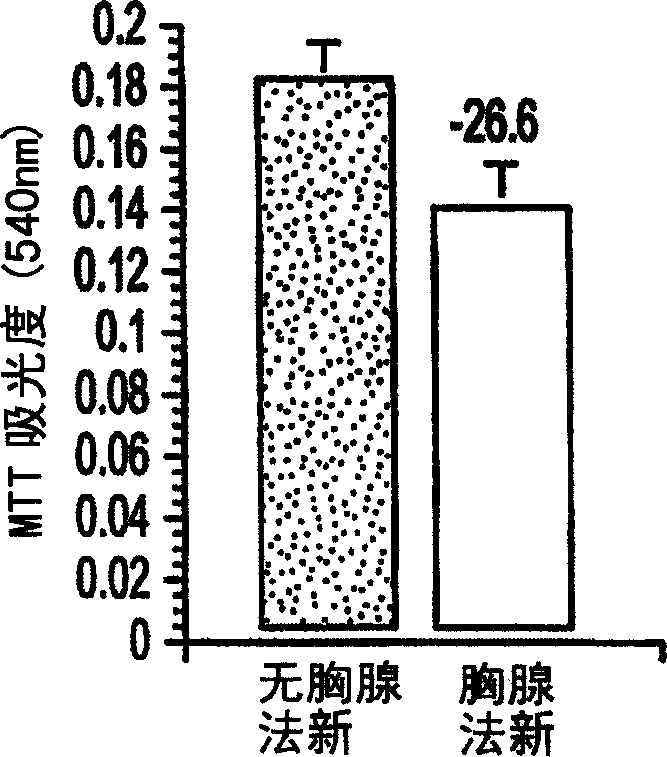
[0029] Thymosin a1 treatment: for acute treatment of thymus fasin, the cells were planted in a 96-well culture plate with a cell density of 20,000 cells / well, and 10 -5 M's thymus was treated with fasin for 24 hours. Cells chronically treated with thymusfasin were inoculated in culture flasks, and during the treatment period (3 days) every 24 hours, 10 -5 M's thymus fasin (added to fresh medium) was treated. In addition, to determine the dose-response curve of 9L cells to thymofasin, cells wer...
Embodiment 2
[0037] Example 2: Study on telomerase-induced apoptosis
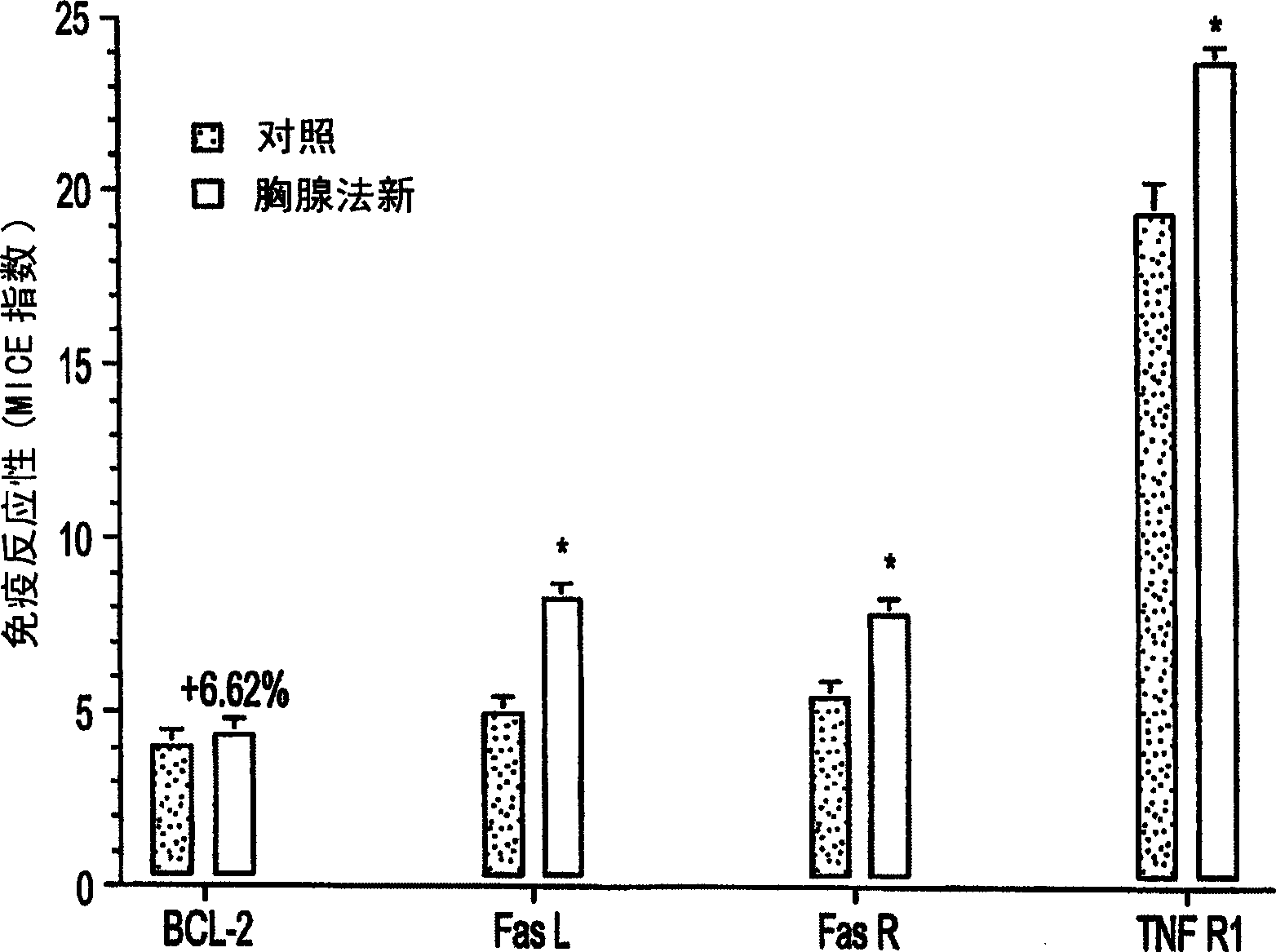
[0038] The above MICE detection study confirmed that thymofasin induced the expression of TNF-R1, FasL and FasR in 9L glioblastoma. In order to determine the difference in the sensitivity of 9L glioblastoma cells treated (or untreated) with thymus method to apoptosis induced by cytotoxic T-lymphocytes (CTL), the following experiments were performed: 9L cells were treated with Thymofasin was treated acutely for 24 hours and chronically treated for 72 hours, then the cells were collected and re-seeded in 96-well black culture plates (7.5×10 5 cells / 75 μl / well), and then contacted with 20,000 units / ml streptolysin O (SLO) plus 100 ng recombinant telomerase B (reaction volume 100 μl) at 37° C. for 1 or 3 hours. Cells were permeabilized with SLO instead of perforin and assays were normalized using recombinant telomerase B. Control studies included parallel reactions with no SLO, no telomerase B, or both. Using ATPlite for...
Embodiment 3
[0040] Example 3: Effect of Thymalfasin on Cell Viability of Primary Neuronal Cell Cultures
[0041] Primary cortical neuronal cell cultures were studied to determine doses of thymofasin that were not toxic to non-neoplastic brain cells. Since the previous studies have shown that as the cell density increases from 1×10 per well 4 up to 5×10 5 The absorbance of CV and MTT increases linearly with the change (de la Monte, 2001 & 2000). Therefore, crystal violet (CV) and MTT assays are used to measure cell viability and mitochondrial function.
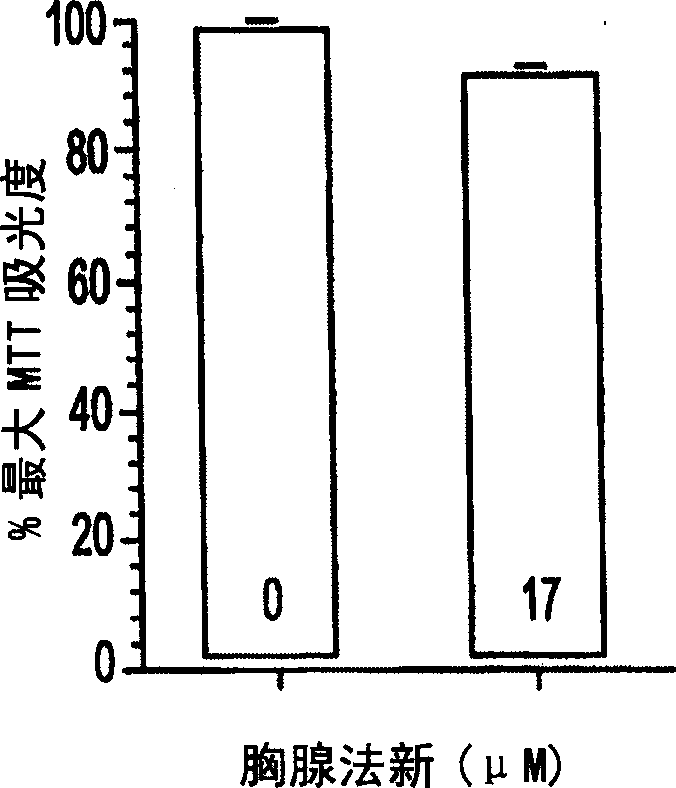
[0042] Use serially diluted thymus fasin (final concentration at 3.3×10 -5 M to 1×10 -9 M) treated primary neuronal cortical cells in 96-well culture plates and found no decrease in cell viability at the experimental doses used. It can be found by MTT assay that the highest concentration of thymus fasin (3.3×10 -7 M) did reduce viability by 30%. However, this dose is higher than the experimental and clinical doses used.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com