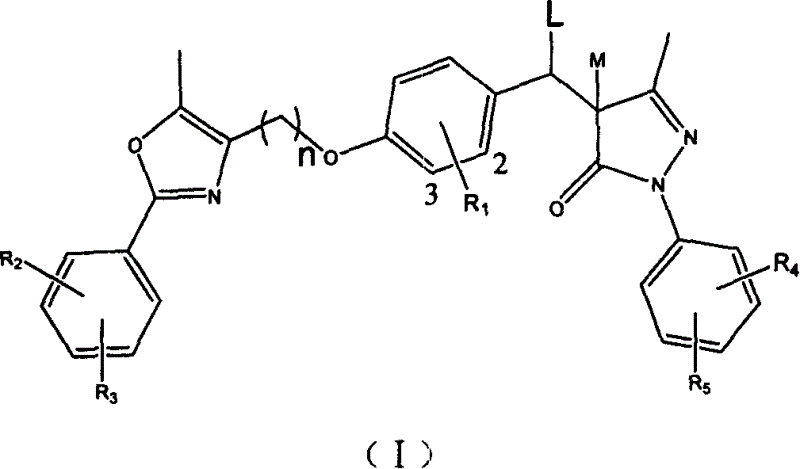
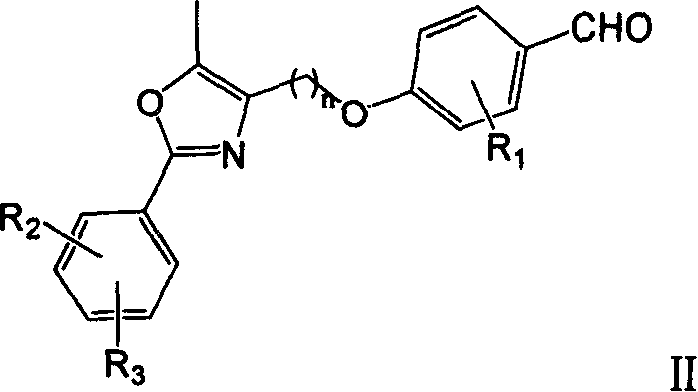
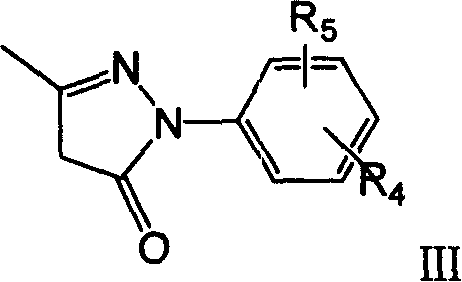
Substituted pyrazolone derivatives and their preparing process and pharmaceutical conpositions
A technology of compounds and medicinal salts, applied in the field of substituted pyrazolone derivatives and their preparation and pharmaceutical compositions, can solve the problem of hypoglycemia and side effects of sulfonylureas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0054] 3-Methyl-1-phenyl-4-[4-(5-methyl-2-phenyl-4-oxazolyl)methoxy]-benzylidene-2-pyrazoline-5- Preparation of ketones (1):
[0055] a. Preparation of 4-(5-methyl-2-phenyl-4-oxazolyl)methoxy-benzaldehyde (II): 5.50g (26.5mmol) 4-chloromethyl-5-methyl -2-Phenyl-oxazole (Compound IV, R 2 =R 3 =H. Refer to the literature Ber., 1915, 48: 897 and Chem. Pharm. Bull., 1971, 19(10): 2050. ), 3.23g (26.5mmol) of p-hydroxybenzaldehyde, 7.32g (53mmol) of potassium carbonate and 80ml of DMF were mixed, and stirred at 80°C for 3h. After cooling, water was added to dissolve unreacted potassium carbonate, and extraction was performed twice with ethyl acetate. The organic phases were combined, washed with water until neutral, and finally washed with saturated brine, and dried with anhydrous sodium sulfate. Concentrate to obtain crude product. The crude product was soaked in 2.5mol / L NaOH solution for 30min, filtered, washed with water until neutral, and dried under infrared light to obtain 7.51g...
example 2
[0062] 3-Methyl-1-phenyl-4-[4-(5-methyl-2-phenyl-4-oxazolyl)methoxy]-benzyl-2-pyrazoline-5-one( 2) Preparation: 1.2g (2.7mmol) of compound (I, R 1 =R 2 =R 3 =H) Dissolve in 20ml of dioxane, use 0.06g of 5% Pd / C as a catalyst, and stir with hydrogen at room temperature and pressure until no more hydrogen is absorbed. The reaction mixture was filtered, the catalyst was recovered, and the filtrate was concentrated and recrystallized from anhydrous methanol. After the crystals are completely precipitated, they are filtered, washed with a small amount of methanol, and dried under an infrared lamp to obtain 0.48 g of white solid, with a yield of 89.4%, mp 137-138°C.
[0063] IR(cm -1 ): 2922
[0064] ESI-MS: 452[M+H] + , 474[M+Na] + ;
[0065] Anal.C 28 H 25 N 3 O 3 Found(%) C74.25 H5.64 N9.03
[0066] Calcd(%)C74.48 H5.58 N9.31
example 3
[0068] 3-Methyl-1-phenyl-4-[3-methoxy-4-(5-methyl-2-phenyl-4-oxazolyl)methoxy]-benzylidene-2- Preparation of pyrazoline-5-one (3):
[0069] a. Preparation of 3-methoxy-4-(5-methyl-2-phenyl-4-oxazolyl) methoxy-benzaldehyde: refer to the preparation method of a in Reference Example 1 from 4-chloromethyl 5-methyl-2-phenyl-oxazole and vanillin prepared. The product is a light yellow solid, with a yield of 72.1%, mp 120-122°C.
[0070]b. Refer to the preparation method of Example 1b. The product is orange-red solid, with a yield of 78.8%, mp192~193°C.
[0071] IR(cm -1 ): 1675(C=O);
[0072] 1 HNMR(500Hz, CDCl 3 ), δ(ppm): 2.35(s, 3H, CH 3 ), 2.46(s, 3H, CH 3 ), 4.03(s, 3H, CH 3 ), 5.18(s, 2H, CH 2 ), 7.17 ~ 7.21 (m, 2H, Ar-H), 7.33 (s, 1H, CH), 7.39 ~ 7.46 (m, 5H, Ar-H), 7.69 ~ 7.72 (q, 1H, Ar-H), 7.94~7.97(d, 2H, Ar-H), 8.00~8.03(q, 2H, Ar-H), 9.01~9.02(d, 1H, Ar-H);
[0073] Anal.C 29 H 25 N 3 O 4 Found(%) C71.78 H5.10 N8.29
[0074] Calcd(%) C72.64 H5.25 N8.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com