Axial substituted phthalocyanine compound, its preparation and application in optical kinetic treatment
A complex and axial technology, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and compounds containing elements of Group 3/13 of the periodic table, etc., which can solve the problems of complex synthesis and high cost , to achieve the effects of strong photosensitization ability, no isomers, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
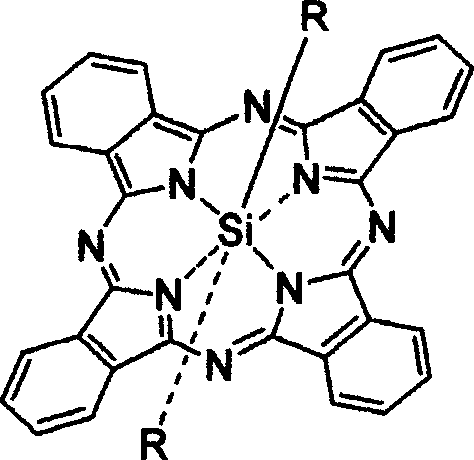
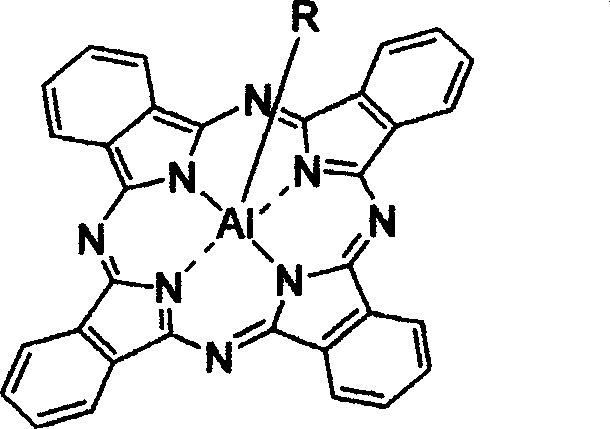
[0043] 2. The preparation method of the new axially substituted phthalocyanine complex of the present invention comprises the following major steps: (a) dichlorosilicon phthalocyanine (or a chloroaluminum phthalocyanine) and the corresponding The reactants are dispersed in a suitable solvent according to a certain molar ratio of feed; (b) in the presence of sodium hydride or potassium carbonate, reflux reaction for 1 hour to 3 days, and monitor the reaction end point by chromatography; (c) by solvent method And / or chromatography to remove excess raw materials and impurities and purify the target product. For the schematic diagram of the reaction, please refer to the attached Figure 19 , 20 .
[0044] The corresponding reactants containing substituents described in the present invention include: sulfamic acid; Glucose; Diethylene glycol butyl ether; 4-ethyl phenol; 4-butyl phenol; 4-aminophenol; 2-ethoxy-4-formylphenol; 2-dimethylaminoethanol; 3-diethylaminophenol; pyrimidi...
Embodiment 1
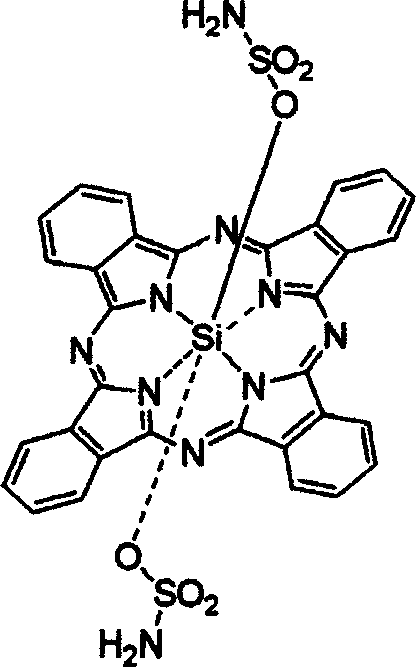
[0048] Stir 0.1g of dichlorosilicon phthalocyanine, 0.3g of sulfamic acid, and 20mg of NaH in 10ml of toluene, reflux for 3 days, remove the solvent by rotary evaporation under reduced pressure, add 100ml of water to the solid, filter, and dissolve the filter cake with dichloromethane, pass Silica gel chromatography column separation and purification, the eluent was ethanol, and after vacuum drying, dark blue bis(aminosulfonyl)silyl phthalocyanine was obtained with a yield of 38.3%.
Embodiment 2
[0050] Stir 0.1g of dichlorosilicon phthalocyanine, 10ml of diethylene glycol butyl ether, and 20mg of NaH in 10ml of toluene, reflux for 3 days, remove the solvent by rotary evaporation under reduced pressure, add 100ml of water to the solid, filter, and dissolve the filter cake with dichloromethane , separated and purified by silica gel chromatography, the eluent is chloroform. After vacuum drying, dark blue bis(2-(2-butoxyethoxy)ethoxysilylphthalocyanine was obtained with a yield of 21.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com