Production method and use for imidacloprid artificial hapten, artificial antigen and specific antibody
An artificial hapten and imidacloprid technology, applied in chemical instruments and methods, specific peptides, material inspection products, etc., can solve the problems of cumbersome process, great influence, unsuitable for large-scale sample detection and analysis, etc., and achieve high sensitivity, Strong specificity and easy on-site monitoring effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
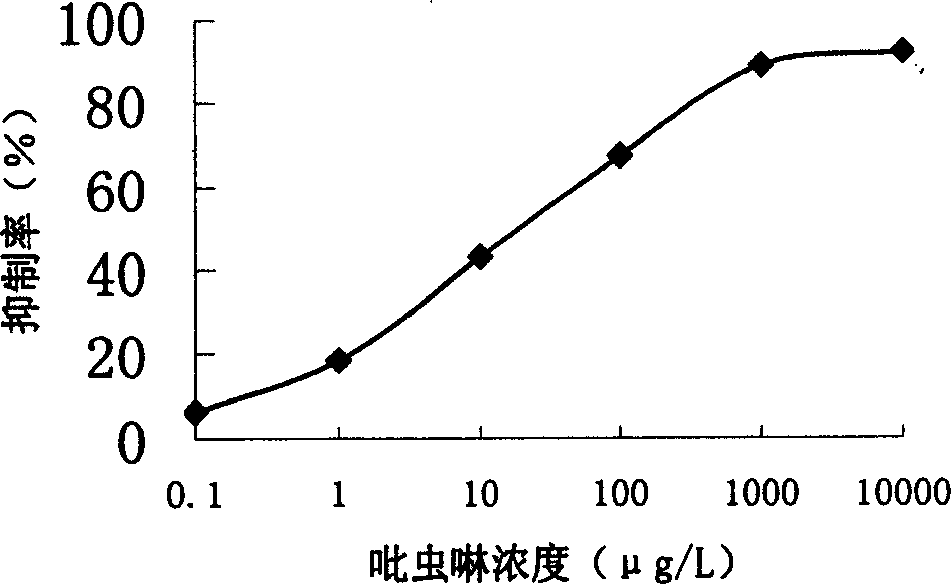
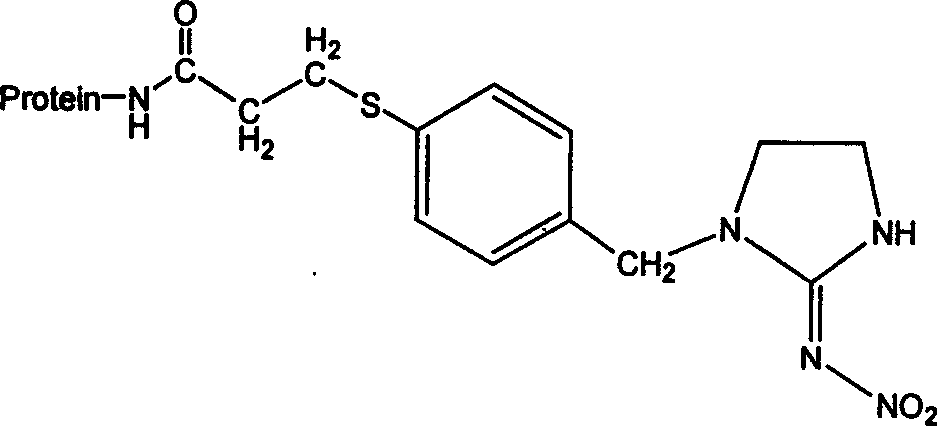
[0037] 3 Antibody preparation and imidacloprid enzyme-linked immunoassay method establishment
[0038] The steps of the preparation method of imidacloprid-specific antibody are as follows:
[0039] 1) The experiment selects healthy male rabbits or mice about half a year old and weighing 2-3 kg. The experimental immunization dose is 0.25-2.0 mg / kg for basic immunization, and 0.5-2.0 mg / kg for booster immunization. Dilute appropriate amount of artificial antigen complexes with normal saline respectively, add an equal volume of Freund's complete adjuvant, fully emulsify until instilled Emulsion droplets do not disperse in water. A combination of subcutaneous multi-point injection on the back and intramuscular injection on the thigh was used. Back subcutaneous immunization at 4 to 6 points, thigh intramuscular injection at 2 to 4 points, booster immunization after 3 to 4 weeks, and then booster immunization every 2 weeks, using incomplete Freund's adjuvant for booster immunizati...
Embodiment 1
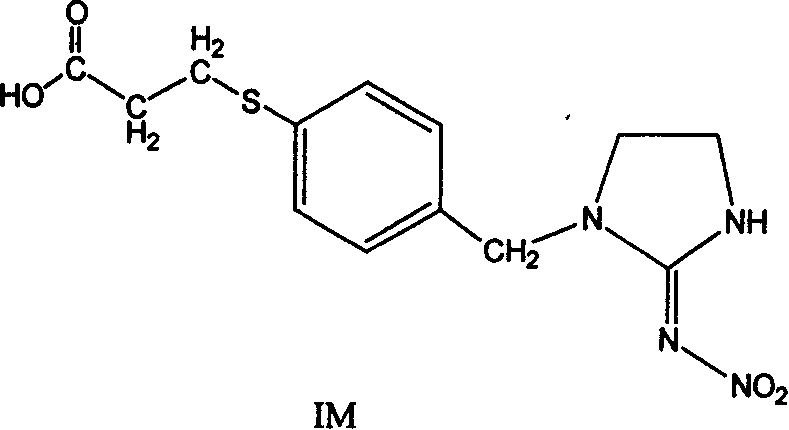
[0043] Example 1: Synthesis of Hapten IM
[0044] According to the feeding ratio of imidacloprid: 3-mercaptopropionic acid as 1: 1, 5.15g (20mmol) of imidacloprid (20mmol) was dissolved in 20mL dimethyl sulfoxide, put into a three-necked flask, and then 2.25g (40mmol) of KOH was added, and magnetically stirred to dissolve it, weighing Get 2.10g (20mmol) of β-mercaptopropionic acid and dissolve it in 10mL dimethyl sulfoxide and transfer it to a constant pressure dropping funnel, slowly drop the β-mercaptopropionic acid solution into a three-necked flask, and slowly heat the oil bath to 100°C. After keeping warm for 2 hours, remove the oil bath. After the reaction solution is naturally cooled to room temperature, add 20 mL of water and adjust the pH value to 3.0-5.0 with dilute hydrochloric acid; extract with 3×30 ml of dichloromethane, collect the organic phase, and wash the organic phase with 3×15 ml of water. phase, dried over anhydrous sodium sulfate, and concentrated to ob...
Embodiment 2
[0048] Example 2: Synthesis of Hapten IM
[0049] According to the feeding ratio of imidacloprid: 3-mercaptopropionic acid as 1:2, 5.15g (20mmol) of imidacloprid (20mmol) was dissolved in 20mL dimethyl sulfoxide, put into a three-necked flask, and then 4.5g (80mmol) of KOH was added, and magnetically stirred to dissolve it, weighing Get 4.2g (40mmol) of β-mercaptopropionic acid and dissolve it in 20mL dimethyl sulfoxide and transfer it to a constant pressure dropping funnel, slowly drop the β-mercaptopropionic acid solution into the three-necked flask, and slowly heat the oil bath to 100°C. After keeping warm for 2 hours, remove the oil bath. After the reaction solution is naturally cooled to room temperature, add 40mL of water and adjust the pH value to 3.0-5.0 with dilute hydrochloric acid; extract with 3×30ml of dichloromethane, collect the organic phase, and wash the organic phase with 3×15ml of water. phase, dried over anhydrous sodium sulfate, and concentrated to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com