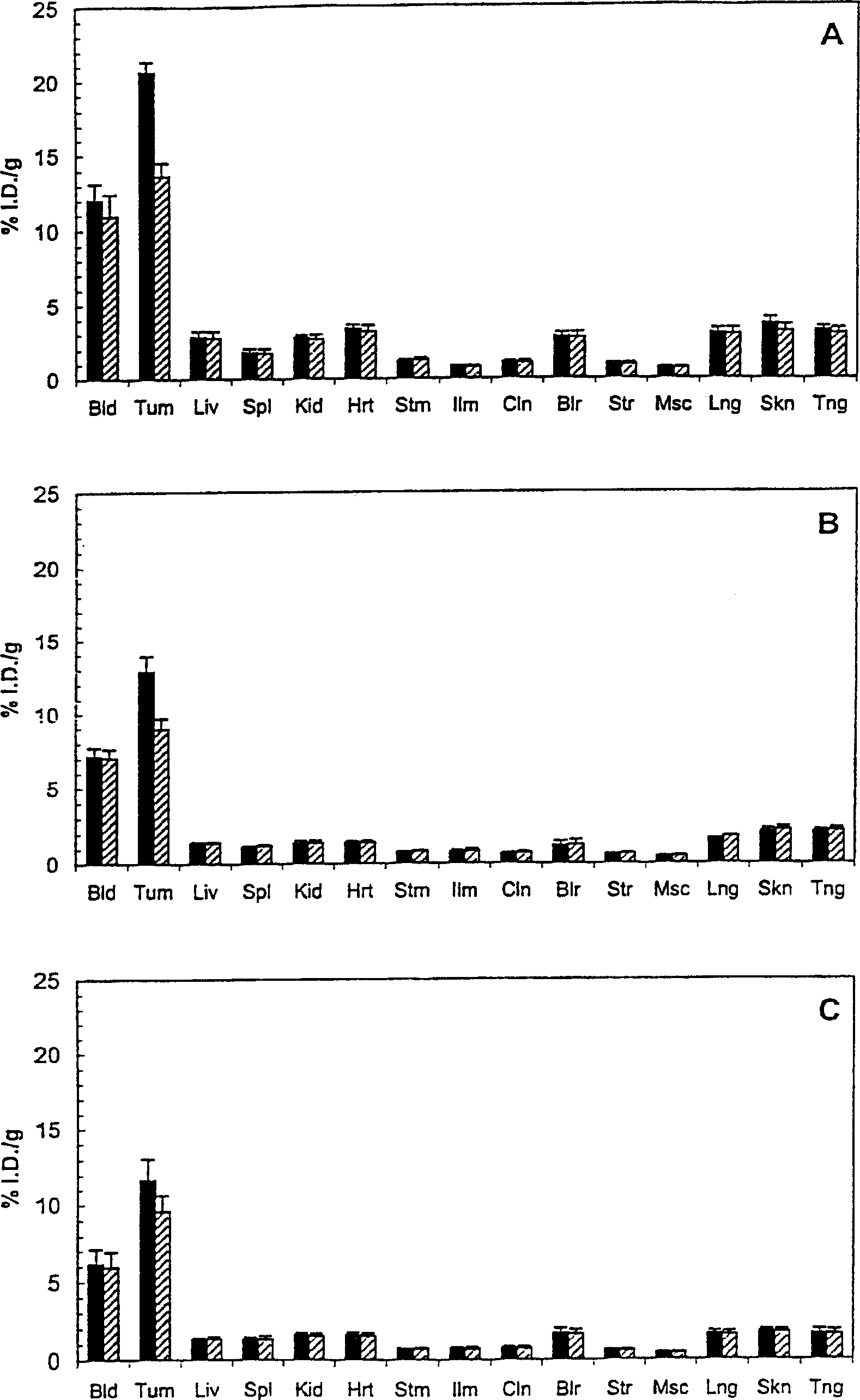
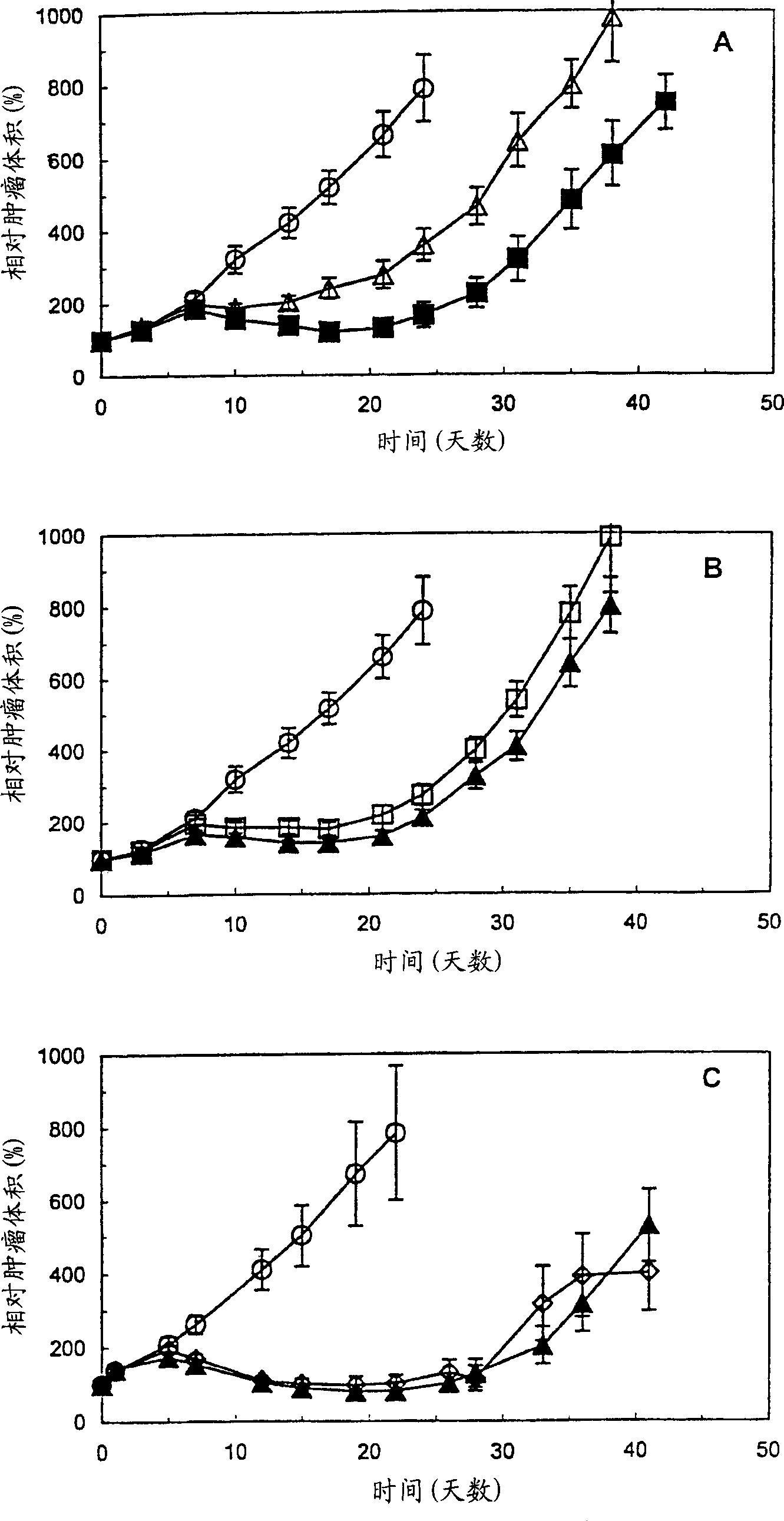
Antibodes specific for CD44V6
A technology of antibodies and antibody molecules, applied in the field of oncology, can solve problems such as poor affinity CDR-grafted antibodies, which cannot be regarded as useful
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] Embodiment 1 radioimmunotherapy
[0148] Materials and methods
[0149] Monoclonal antibodies. mMAb BIWA 1=VFF 18 (secreted by a hybridoma cell line, deposited at DSM-Deutsche Sammlung furMikroorganismen und Zellkulturen GmbH, Mascheroder Weg 1b, D-38124 Braunschweig, Deutschland on June 7, 1994 under accession number DSM ACC2174; see also WO 95 / 33771) was produced by immunizing BALB / c mice with a glutathione S-transferase fusion protein containing human CD44 domain v3-v10 (Heider et al., 1996). The epitope recognized by BIWA 1 has been assigned to amino acids 360-370 in CD44 domain Vb (numbering according to Kugelman et al. (1992)). The batch used in this study was obtained after protein-G Sepharose purification and dialysis against PBS.
[0150] MAb U36 (IgG1) was derived after immunization of mice with the HNSCC cell line UM-SCC-22B and recognizes a different epitope in CD44v6 than that recognized by BIWA 1. U36 was purified from concentrated tissue culture super...
example 2
[0215] Example 2 sequence details
[0216] This example shows the detailed sequence, such as the location of the cloning site, leader sequence and untranslated regions.
[0217] abbreviation:
[0218] aa = amino acid
[0219] nt = nucleotide sequence
[0220] SEQ ID No.1 VH BIWA 4 / 8aa
[0221] EVQLVESGGGLVKPGGSLRLSCAASGFTFSSYDMSWVRQAPGKGLEWVSTISSGGSY
[0222] TYYLDSIKGRFTISRDNAKNSLYLQMNSLRAEDTAVYYCARQGLDYWGRGTLVTVSS
[0223] SEQ ID No.2 VL BIWA 4aa
[0224] EIVLTQSPATLSSLSPGERATLSCSASSSINYIYWYQQKPGQAPRLLIYLTSNLASGVPAR
[0225] FSGSGSGTDFTLTISSLEPEDFAVYYCLQWSSNPLTFGGGTKVEIK
[0226] SEQ ID No.3 VL BIWA 8aa
[0227] EIVLTQSPATLSSLSPGERATLSCSASSSINYIYWLQQKPGQAPRILIYLTSNLASGVPARF
[0228] SGSGSGTDFTLTISSLEPEDFAVYYCLQWSSNPLTFGGGTKVEIK
[0229] SEQ ID No.4 VH BIWA 4 / 8nt
[0230] GAAGTGCAGCTGGTGGAGTCTGGGGGAGGCTTAGTGAAGCCTGGAGGGTCCCTAAGACTCTCC
[0231] TGTGCAGCCTCTGGATTCACTTTCAGTAGCTATGACATGTCTTGGGTTCGCCAGGCTCCGGGG
[0232] AAGGGGCTGGAGTGGGTCTCAAACCATTAGTAGTGGTGGTAGTTACAC...
example 3
[0698] Example 3 Clinical Research 1170.1
[0699] 1. List of term abbreviations and definitions
[0700] ADCC antibody-dependent cell-mediated cytotoxicity
[0701] Adverse effects of AEs
[0702] ALT alanine aminotransferase
[0703] a.p.
[0704] AP alkaline phosphatase
[0705] AST aspartate aminotransferase
[0706] AUC is the area under the concentration-time curve
[0707] Bq Becquerel, radioactive Becquerel
[0708] SI unit (1Bq = one decay / second)
[0709] BSA bovine serum albumin
[0710] CD44v6 CD44 variant isoform v6
[0711] CDS Common Drug Safety (Corporate Drug Safety)
[0712] Centi Gray detection of cGy versus radioactivity
[0713] CHO Chinese Hamster Ovary Cells
[0714] Ci Curie; unit of radioactivity; 1 Curie=37×10 9 Decay / s
[0715] =37GBq
[0716] CL total body clearance
[0717] cMAb chimeric monoclonal antibody
[0718] cm cm
[0719] C max Maximum Drug Concentration Observed
[0720] cpm counts ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com