Oculentum for treating eye disease and preparing method thereof
A technology of eye ointment and raw materials, applied in skin diseases, sensory diseases, ointment delivery, etc., can solve the problems of poor absorption, complicated process, complicated prescription, etc., and achieve the effect of fast absorption, simple process and good analgesic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
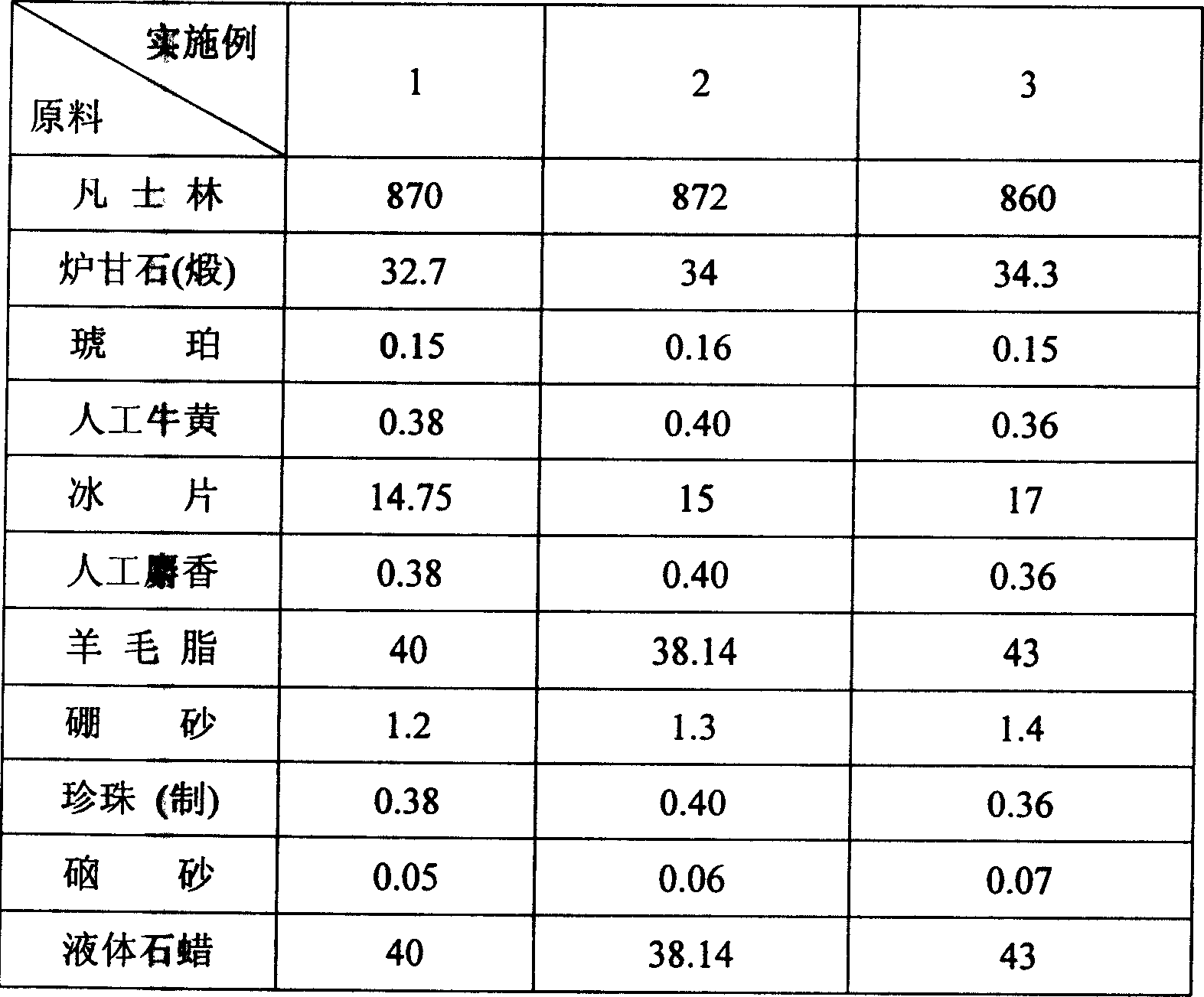
[0023] The dosage of the medicine embodiment of the present invention is as follows (parts by weight):
[0024]
[0025] An eye ointment for treating ophthalmic diseases, the steps are as follows:
[0026] 1. crush
[0027] Carry out fine powder pulverization with pearl, borax respectively, cross 200 mesh sieves;
[0028] 2. Preparation and research
[0029] 2.1 Grind artificial musk, artificial bezoar, borneol and calamine (calcined) with 1 / 5 of the fixed amount, and pass through a 200-mesh sieve;
[0030] 2.2 Grind pearl fine powder, borax fine powder and amber, and pass through a 200-mesh sieve;
[0031] 2.3 Grind the nacre and pass through a 200-mesh sieve;
[0032] 3. Mix
[0033] Mix the drug powders under 2.1, 2.2 and 2.3 evenly;
[0034] 4. Jet crushing
[0035] The mixed medicinal powder is pulverized by a jet mill to obtain the original powder;
[0036] 5. Handling of auxiliary materials
[0037] 5.1 Sterilize the liquid paraffin at a controlled temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com