Sodium ferulic acid osmosis pump controlled release formulation and its preparation method
A technology of osmotic pump controlled release and sodium ferulate, which is applied in pill delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve problems that are difficult to achieve, and improve compliance, reduce the number of medications, blood The effect of stable drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
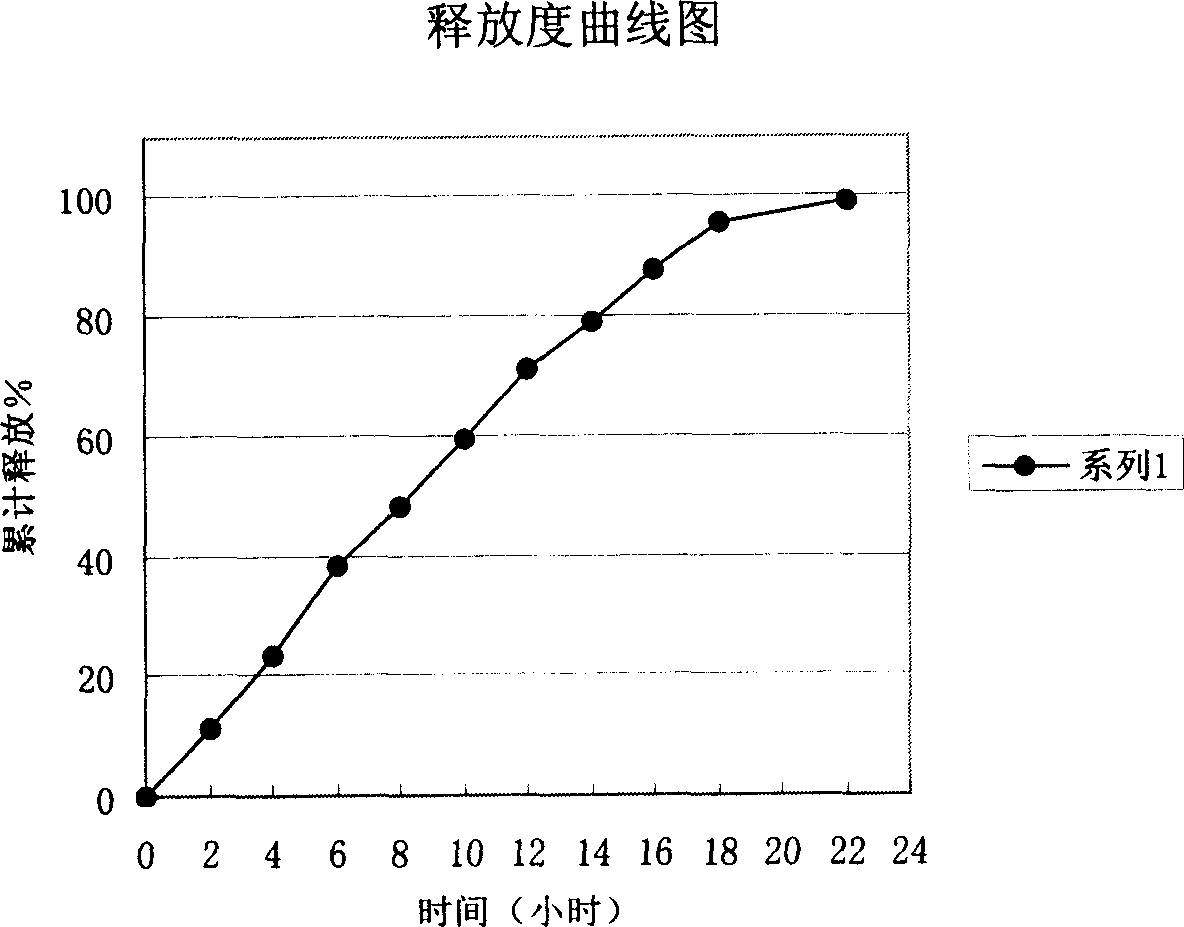
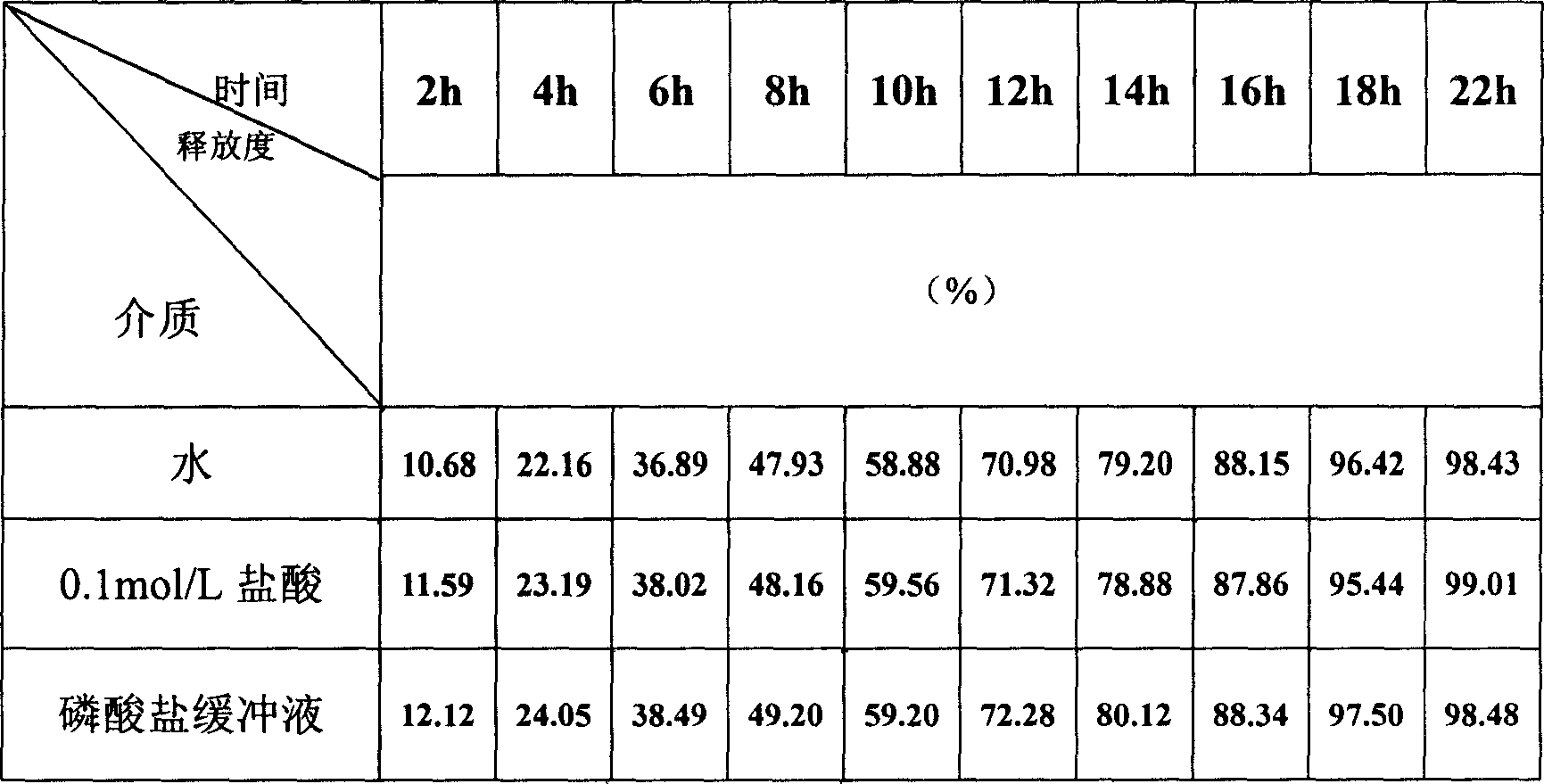
Image
Examples
Embodiment 1
[0036] Tablet prescription:
[0037] Sodium Ferulate 150g
[0039] Croscarmellose Sodium 9g
[0040] Povidone K30 12g
[0041] Magnesium Stearate 1.5g
[0042] Semipermeable Membrane Prescription:
[0043] Cellulose acetate 15g
[0044] Polyethylene Glycol - 1000 3g
[0045] Moisture-proof film prescription Opadry model opadryII configuration method: put the solvent in the container, the solvent is water, and slowly add the Opadry model opadryII into the solvent while stirring, with a solid content of 8%-20%
[0046] Preparation Process:
[0047] Sieve the sodium ferulate, croscarmellose sodium, povidone K30, and magnesium stearate in the prescription separately for later use, crush and sieve the sodium chloride for later use, put the main and auxiliary materials in a container, and mix well , Add lubricant ethanol: water (1:1) to make soft materials. Granulate with a 20-mesh sieve, dry in a drying oven at 45 degrees Celsius, and granulat...
Embodiment 2
[0051] Tablet prescription:
[0052] Sodium Ferulate 1500g
[0053] Sodium chloride 750g
[0054] Sucrose 750g
[0055] Croscarmellose Sodium 90g
[0056] Povidone K300 120g
[0058] Semipermeable Membrane Prescription:
[0059] Cellulose acetate 150g
[0060] Polyethylene Glycol-4000 20g
[0061] Moisture-proof film prescription Opadry model opadryII configuration method: put the solvent in the container, the solvent is water, and slowly add the Opadry model opadryII into the solvent while stirring, with a solid content of 8%-20%
[0062] Preparation Process:
[0063] Sieve the sodium ferulate, croscarmellose sodium, povidone K30, and magnesium stearate in the prescription separately for later use, crush and sieve the sodium chloride for later use, put the main and auxiliary materials in a container, and mix well , Add lubricant ethanol: water (1:1) to make soft materials. Granulate with a 20-mesh sieve, dry in a drying oven at 45 deg...
Embodiment 3
[0067] Tablet core prescription
[0068] Sodium Ferulate 450g
[0069]Sodium chloride 225g
[0070] Sucrose 225g
[0071] Croscarmellose Sodium 27g
[0072] Povidone K30 36g
[0074] Semipermeable Membrane Prescription:
[0075] Cellulose acetate 45g
[0076] Polyethylene glycol-6000 4.5g
[0077] Moisture-proof film prescription Opadry model opadryII configuration method: put the solvent in the container, the solvent is water, and slowly add the Opadry model opadryII into the solvent while stirring, with a solid content of 8%-20%
[0078] Preparation Process
[0079] Sieve the sodium ferulate, croscarmellose sodium, povidone K30, and magnesium stearate in the prescription separately for later use, crush and sieve the sodium chloride for later use, put the main and auxiliary materials in a container, and mix well , Add lubricant ethanol: water (1:1) to make soft materials. Granulate with a 20-mesh sieve, dry in a drying oven at 45 degr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com