Dihydroporphine E6 zinc compound photosensitizer and its preparation method and application
A technology of chlorin and zinc compound, which is applied in the field of chlorin E6 zinc compound photosensitizer, can solve the problems affecting the distribution and positioning of photosensitizer, and achieve excellent overall performance, less toxic and side effects, and convenient use Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0028] The preparation of embodiment 1 chlorin E6 zinc compound
[0029] Scheme 1. Unstabilized step of chlorin E6
[0030] Reagent:
[0031] Chlorin E6; (C 34 h 36 N 4 o 6 , m.w.596.68),
[0032] Sodium bicarbonate, (NaHCO 3 , m.w.84.01),
[0033] Polyvinylpyrrolidone (PVP), (m.w.10,000),
[0034] step:
[0035] a. Dissolving 15M sodium bicarbonate in deionized water with a concentration of 7mg / ml;
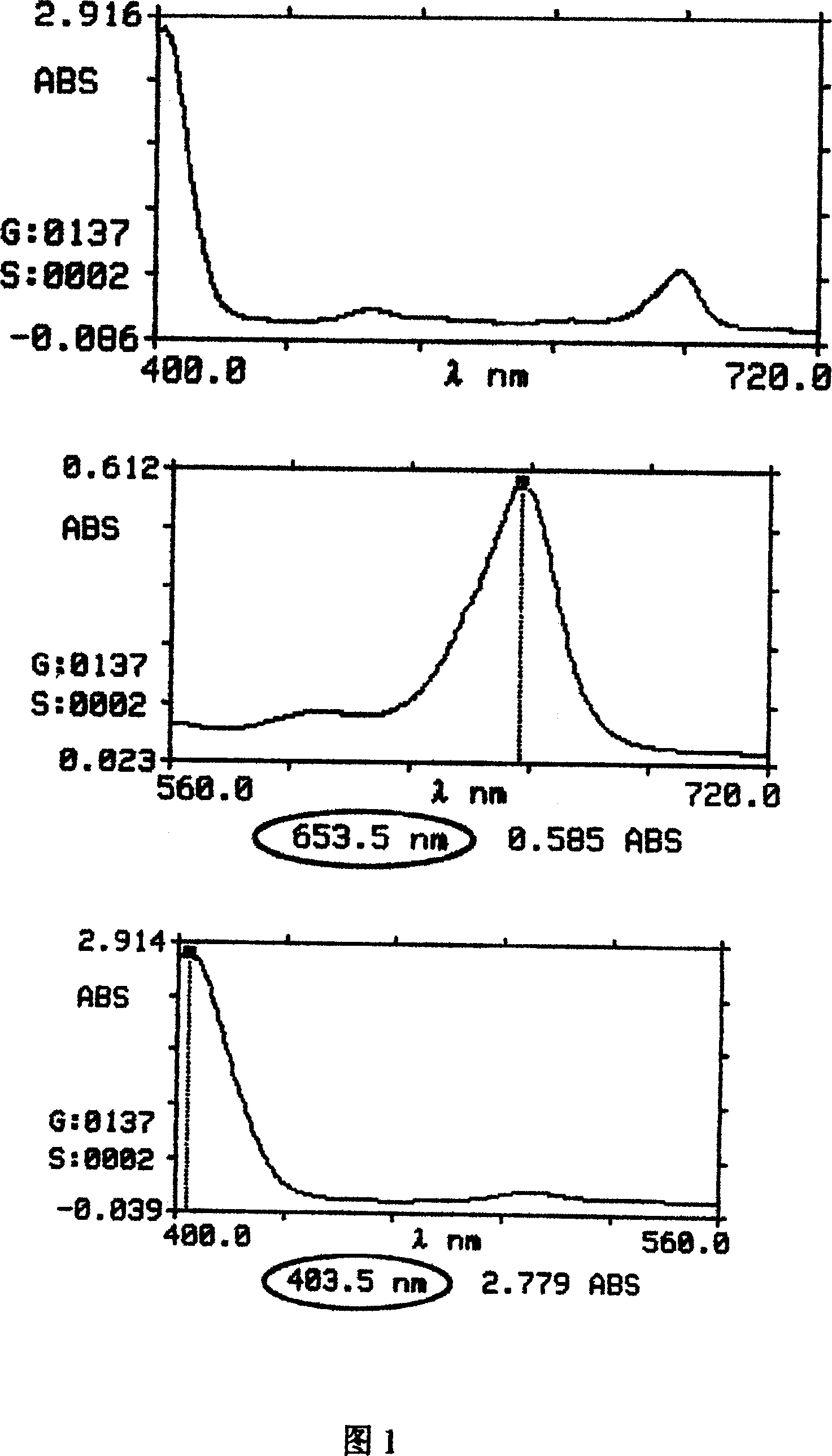
[0036] b. Add 1M chlorin E6, slowly stir the solvent, the absorption spectrum of chlorin E6 is shown in Figure 1;
[0037] c. Dissolve 1M PVP (10000) in deionized water with a concentration of 80mg / ml; then add it to the chlorin E6 test solution and stir slowly at 40°C;
[0038]d. Complete the stabilization of the first step and keep at 40°C for 2 hours with slow agitation.
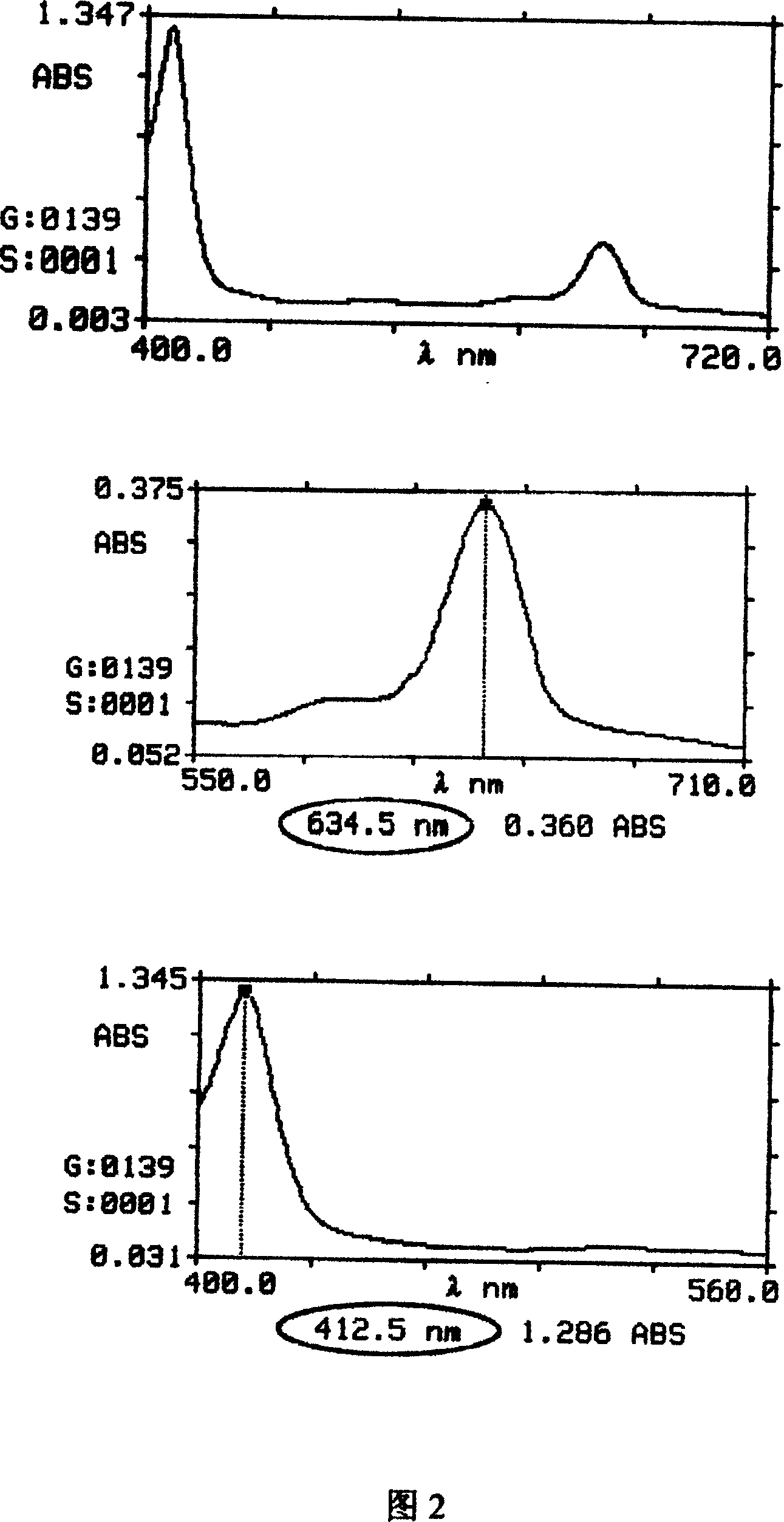
[0039] Process flow 2. the synthetic steps of chlorin E6 zinc compound
[0040] Reagent:
[0041] Unstabilized chlorin E6 (see scheme 1)
Embodiment 2
[0056] Example 2 Pharmacokinetics
[0057] Pharmacokinetic distribution in specific organs, tissues, body fluids and tumor tissue (embriocarcinome) for 30 hours. Test mice: female BALB / c mice with a total weight of 20-21 grams. The study was performed on the basis of a Percin-elmer fluorescence spectrophotometer for intraperitoneal injection of organ and tumor tissues at a dose of 2.5 mg / kg (body weight) of chlorin E6 zinc complex.
[0058] The injection results are shown in Table 1, which shows that:
[0059] A. Animals can well withstand the intraperitoneal injection of chlorin E6 zinc compound at a dose of 2.5 mg / kg without any toxic symptoms. The behavioral responses of the mice are both immediately after injection and 30 hours after injection. No effect.
[0060] B. The prepared dose is rapidly absorbed from the abdomen into the blood and stored in the liver within one hour after injection. Its volume in liver tissue is 10 to 14 times higher than its level in blood. ...
Embodiment 4 2
[0074] Embodiment 4 Chlorin E6 zinc compound produces the comparative experiment of single-bond oxygen
[0075] The chlorin E6 zinc compound photosensitizer of the present invention can generate single bond oxygen under light, and its single bond oxygen yield with existing photosensitizers is shown in Table 2.
[0076] Table 2
[0077] Photosensitizer Photosensitiser
Yield fΔ
Protoporphyrin dme
0.57
Haemotoporphyrin
0.65
Uroporphyrin III Uroporphyrin III
0.52
TPP
0.63
Tetrakis(3-hydroxyphenyl)porphyrin m-THPP
0.46
0.32
Porphycene
0.30
Chlorin e6 Chlorin e6
0.32
Zinc(II) Chlorin e6Zinc(II)Chlorin e6
0.46
[0078] Chlorin E6 zinc complex photosensitizer exhibits luminescence due to single-bond oxygen. It exists in two luminescent forms, but both are relatively weak in aqueous solution. The emission regi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com