Preparation and purification of diborane
A technology of diborane and potassium borohydride, applied in the field of preparation and purification of diborane, can solve problems such as unfavorable lithium borohydride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
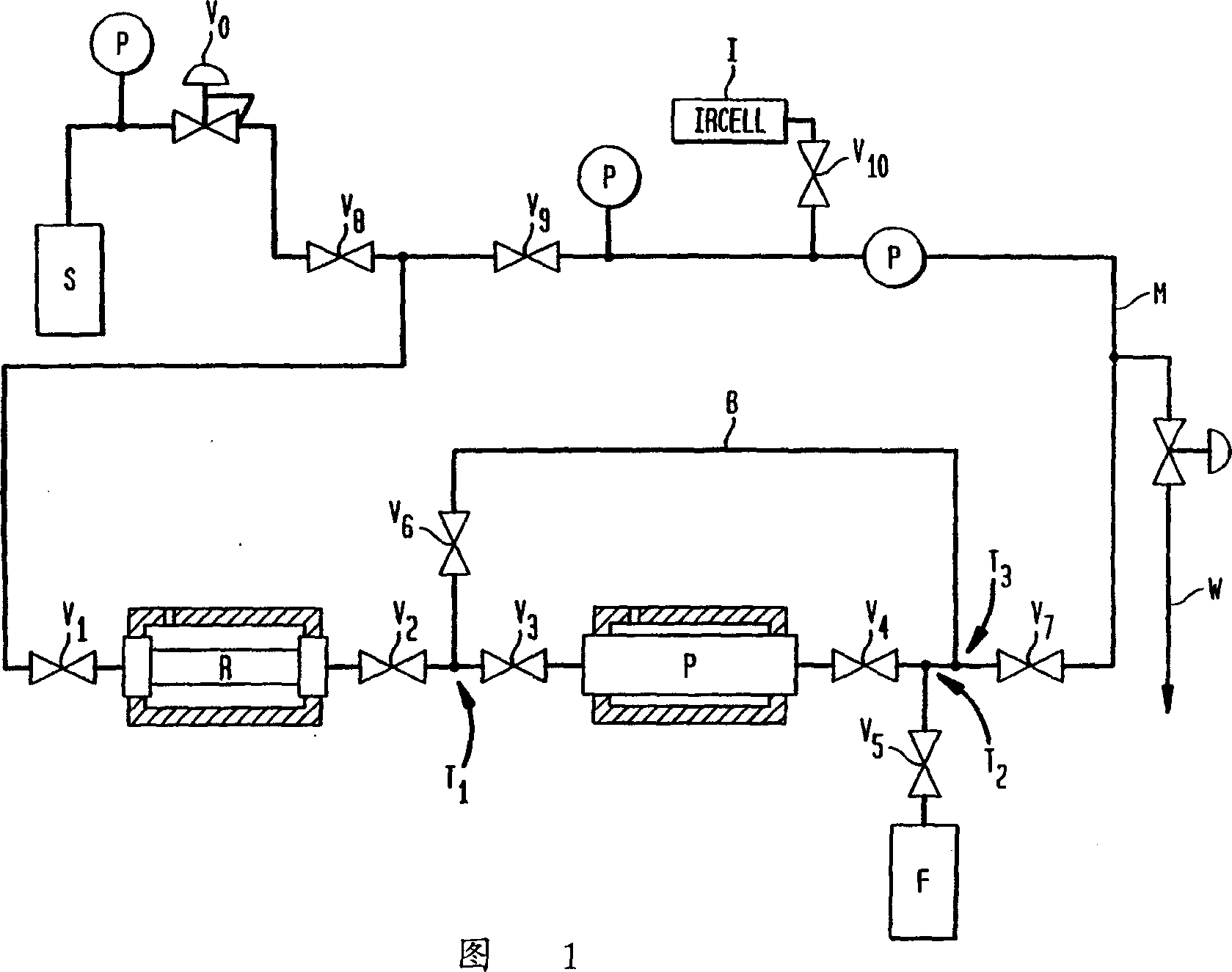
[0026] Fifty grams of potassium borohydride was wrapped in a glove bag under helium and placed in a cylindrical stainless steel reactor R (volume 195 cc) with flanges at each end. The reactor is closed by fitting a flange with a closed valve. The reactor assembly is placed into a jacketed empty cylinder-like vessel. Purifier P is a stainless steel bubbler (volume 972cc) fitted with inclined tubes, with inlet and outlet valves with VCR components welded on top. The purifier is filled more than half full with potassium borohydride pellets through the fill port. Filling of the purifier is performed in a glove bag with helium flow. The fill port is closed with a 1 / 2" VCR cap. Figure 1 shows the reactor and purifier connections. All parts of the apparatus including the reactor and purifier are to be evacuated. To dry the potassium borohydride, heat the purify slightly while evacuating P. The jacketed vessel surrounding the reactor was filled with dry ice. The surrounding of the ...
example 2
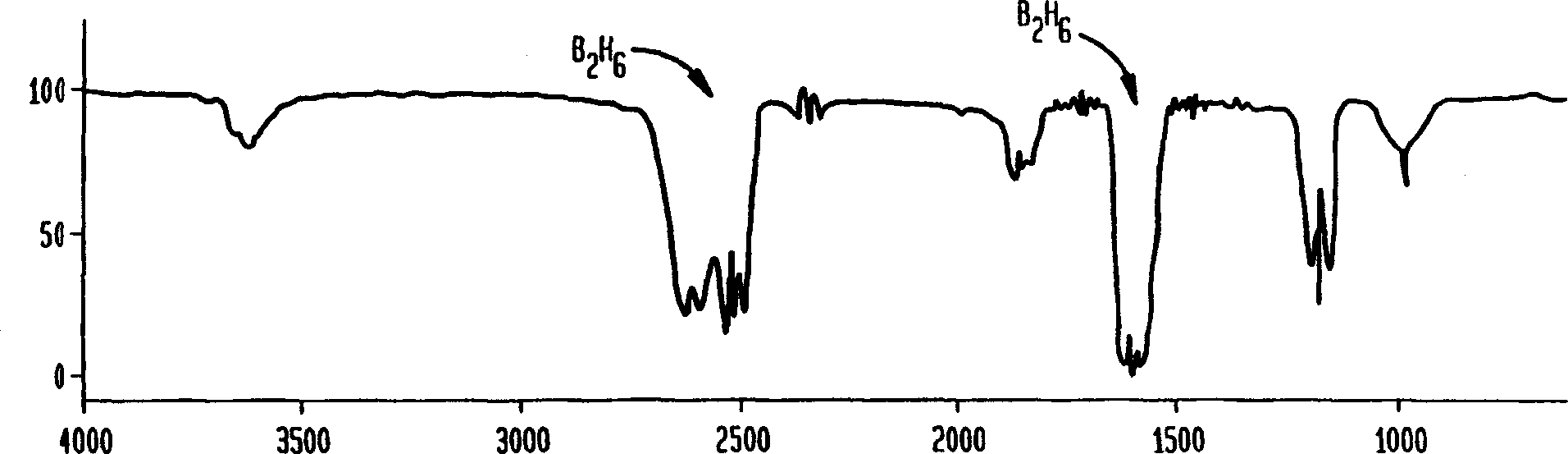
[0028] The procedure of Example 1 was repeated except that the purifier P with potassium borohydride was kept at room temperature. Samples were collected through the purifier up to a pressure of 53 Torr for IR scanning. The spectrum shows only a small amount of diborane and no BF3. However, at a pressure of 14 Torr, a sample containing diborane and some boron trifluoride was collected through bypass line B and through purifier P; the spectrum of this sample was similar to Fig. 2b. These results indicate that the scrubber removes BF3 completely at room temperature, but diborane has some differences. Example 3
example 3
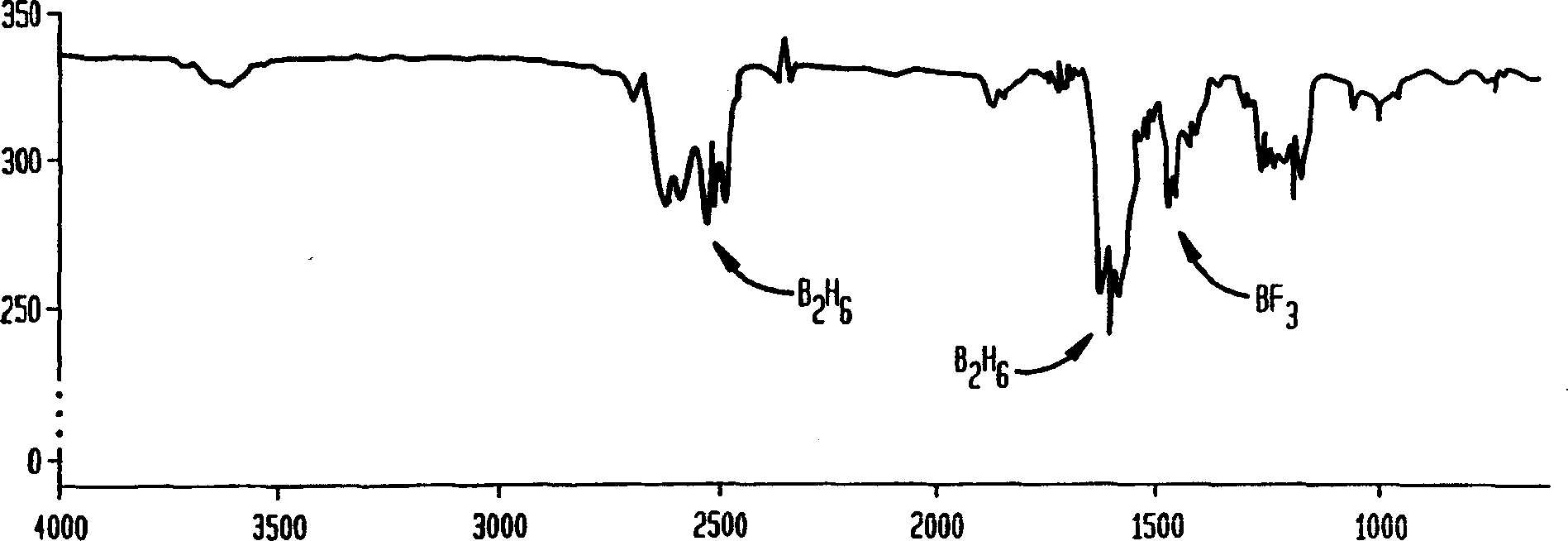
[0029] The procedure of Example 1 was repeated except that the clarifier was filled with soda lime (a mixture of sodium hydroxide, calcium oxide, and calcium hydroxide). Cool the reactor and purifier to dry ice temperature. Samples were collected up to a pressure of 51 Torr from the reactor and passed through the purifier, and samples were collected up to a pressure of 19 Torr bypassing the purifier. Comparing the IR scans of these samples revealed that the scrubber was able to remove boron trifluoride from the diborane / boron trifluoride mixture. However, an imbalance in the conversion of some diborane to noncondensable hydrogen with soda lime was observed. Comparative Example 4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com