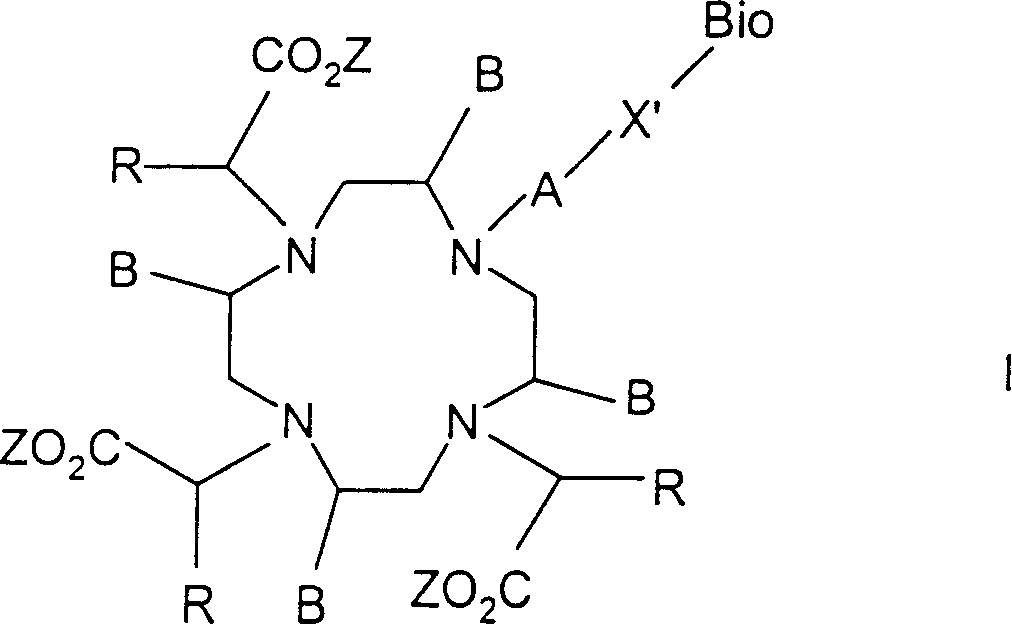
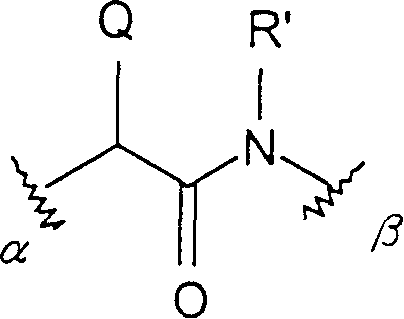
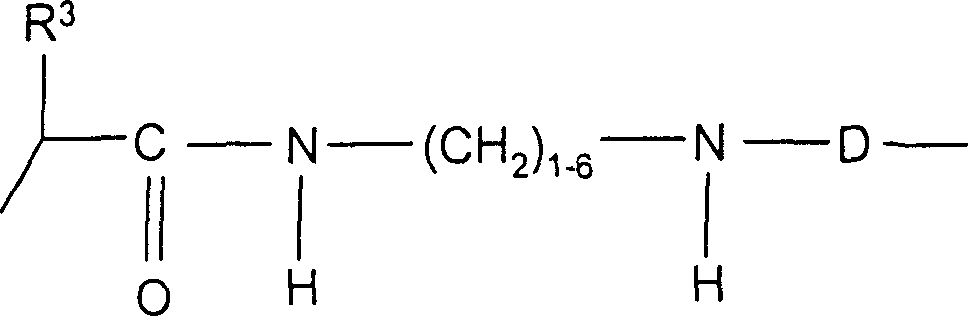Conjugates of macrocyclic metal complexes with biomolucules and utilization thereof for producing agents for use in NMR diagnosis and radiodiagnosis and radiotherapy
A technology of biomolecules and conjugates, which is used in in vivo radioactive preparations, preparations for in vivo experiments, and medical preparations with inactive ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] a) 10-[4-(benzyloxycarbonyl)-1-methyl-2-oxo-3-azetidine]-1,4,7-α, α′, α″-trimethyl- 1,4,7-tris-(benzyloxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane
[0142] 25 g (81.1 mmol) of 2-bromopropionylglycine-benzyl ester (Example 1e of WO 98 / 24774) were added to 27.9 g (162.2 mol) of 1,4,7,10-tetraazacyclododeca The latter had been dissolved in 300 ml of chloroform and then stirred overnight at room temperature. 250 ml of water were added, the organic phase was separated and then washed twice with 200 ml of water each. The organic phase was dried over magnesium sulfate and evaporated to dryness in vacuo. The residue was chromatographed on silica gel (solvent: chloroform / methanol / 25% ammonia=10 / 5 / 1). The thus obtained 1-[4-(benzyloxycarbonyl)-1-methyl-2-oxo-3-azetibutyl]-1,4,7,10-tetraazacyclododecane ( 19.6 g; 50 mmol; 62% of theory) and a solution of 60 ml (0.35 mol) of N-ethyldiisopropylamine in 200 ml of dichloromethane were added to 62.45 g (0.2 mol) of 2-(trifluor...
Embodiment 2
[0161]a) 10-[4-(Benzyloxycarbonyl)-1-methyl-2-oxo-3-azetidine]-1,4,7-α,α′,α″-tri(isopropyl base)-1,4,7-tris(benzyloxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane
[0162] 19.6 g (50 mmol) of 1-[4-(benzyloxycarbonyl)-1-methyl-2-oxo-3-azetibutyl]-1,4,7, which was used as an intermediate in Example 1a , a solution of 10-tetraazacyclododecane and 60ml (0.35mol) of N-ethyldiisopropylamine in 200ml of dichloromethane was added to 68.1g (0.2mol) of 2-(trifluoromethanesulfonyloxy A solution of benzyl isovalerate (Walker et al., Tetrahedron (1997), 53(43), 14591) in 400 ml of dichloromethane was stirred at reflux for 6 hours and then at room temperature overnight. Each was extracted 3 times with 500 ml of water, the organic phase was dried over magnesium sulphate and evaporated to dryness. The residue is chromatographed on silica gel (solvent: dichloromethane / methanol: 20 / 1). Fractions containing product were combined and concentrated by evaporation.
[0163] Yield: 33.7 g (70% o...
Embodiment 3
[0181] a) 10-[4-(benzyloxycarbonyl)-1-methyl-2-oxo-3-azetidine]-1,4,7-α,α′,α″-tri(cyclohexyl )-1,4,7-tris(benzyloxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane
[0182] 19.6 g (50 mmol) of 1-[4-(benzyloxycarbonyl)-1-methyl-2-oxo-3-azetibutyl]-1,4,7, which was used as an intermediate in Example 1a , a solution of 10-tetraazacyclododecane and 60ml (0.35mol) of N-ethyldiisopropylamine in 200ml of dichloromethane was added to 76.1g (0.2mol) of 2-(trifluoromethanesulfonyl Oxy)-benzyl 2-cyclohexyl acetate (Qabar et al., Tetrahedron Letters (1998), 39(33), 5895) in a solution in 400 ml of dichloromethane was stirred at reflux for 6 hours and then at room temperature overnight. Each was extracted 3 times with 500 ml of water, the organic phase was dried over magnesium sulfate and evaporated to dryness. The residue is chromatographed on silica gel (solvent: dichloromethane / methanol: 20 / 1). Fractions containing product were combined and concentrated by evaporation.
[0183] Yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com