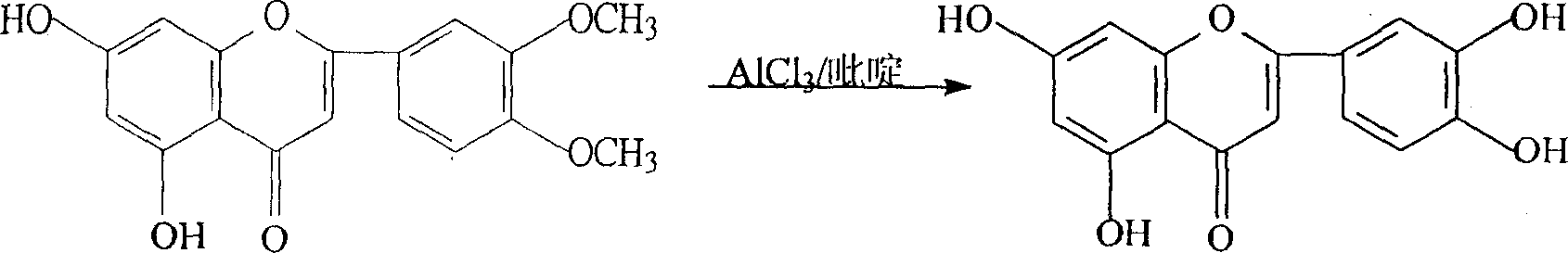Process for synthesizing luteolin
A technology of luteolin and reaction temperature, applied in the production of bulk chemicals, organic chemistry, etc., can solve problems such as difficulty in realizing large-scale production, affecting product quality, inappropriateness, etc., achieving convenient operation, guaranteed product quality, and reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment one, the preparation of luteolin
[0020] Add 30Kg of 3',4'-dimethoxy-5,7-dihydroxyflavone and 120Kg of pure anhydrous pyridine into the enamel reaction kettle, stir to dissolve, heat up the temperature slowly to about 100°C, divide Add aluminum trichloride in batches, a large amount of heat is released, the temperature is controlled not to exceed 180°C, and the addition of aluminum trichloride is completed in about 1 hour. After the addition, react at 160°C-180°C for 3-5 hours, track whether the demethylation is complete by high performance liquid phase, and determine the reaction time.
[0021] Pump 1000Kg of water into the hydrolysis kettle, pre-cool to 10°C, put the above-mentioned reactant into the hydrolysis kettle while it is hot, stir, remove the gas, cool, and separate the crude product in the centrifuge. Wash with 50Kg cold water, wash with DMF / H 2 O and 95% ethanol were recrystallized twice to obtain 22.1Kg of luteolin, with a yield of 81%, and a...
Embodiment 2
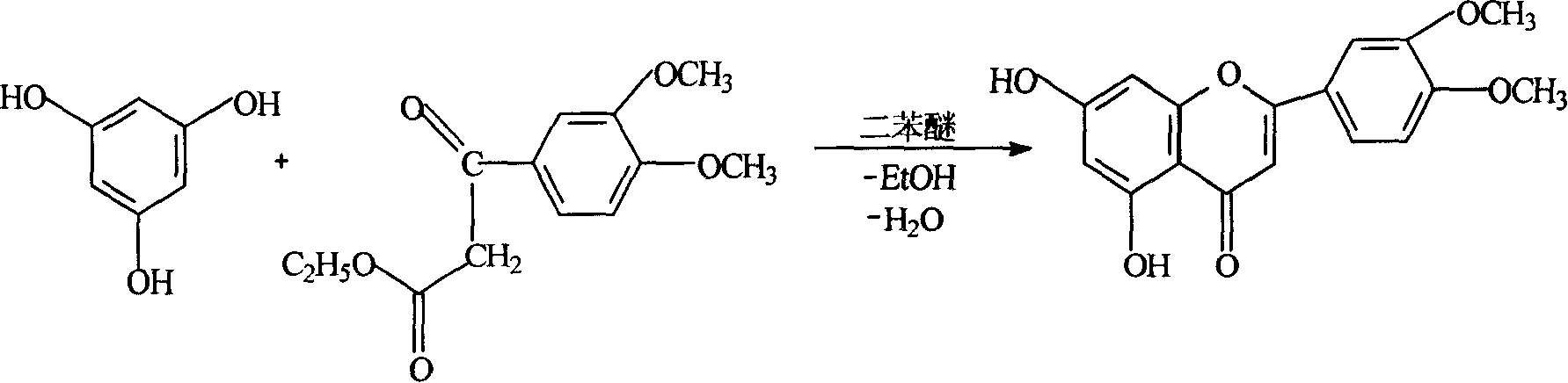
[0022] Embodiment two, 3', the preparation of 4'-dimethoxy-5,7-dihydroxyflavone
[0023] Add 20Kg of phloroglucinol and 60Kg of ethyl 3,4-dimethoxybenzoylacetate into the enamel reaction kettle, stir, dissolve, heat, and raise the temperature to 140°C-160°C, under vacuum (1330Pa) pressure reduction Reflux for 2 hours, put 80Kg of diphenyl ether in a little cold, and then reflux reaction at 140°C-160°C for 4-5 hours under vacuum (6600Pa) under reduced pressure, cool, and when cooled to 30°C, filter with suction, soak in 40Kg95% ethanol for 30 Minutes, beating, centrifuge filter to obtain 21Kg of yellow crude product of 3',4'-dimethoxy-5,7-dihydroxyflavone. The mother liquor was cooled, and after standing still, a small amount of crude product was obtained by filtration, with a yield of 42.2%, and HPLC≥92%. The crude product was used in the demethylation reaction without further purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com