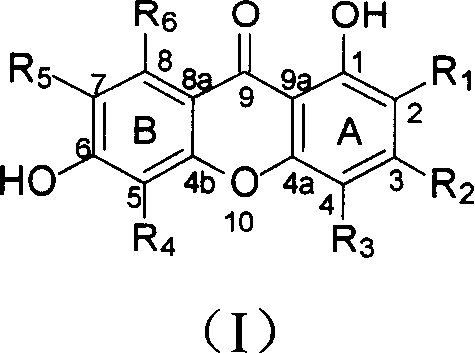
Isopentenyl xanthone compounds and their use in the preparation of antitumor medicines
A technology of isopentenyl ketone and tumor drug, which is applied in the field of traditional Chinese medicine and can solve the problems of unknown anti-tumor active ingredients of Zheshu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Extracting and Separating New Prenyl Oxonone Compounds from Zheshu
[0037]Dry and crush 13.6kg of the root of C.tricuspidata (Carr.) Bur., extract with 95% ethanol, concentrate the extract under reduced pressure to obtain extract, suspend the extract with water, and extract with petroleum ether and chloroform in turn , to obtain petroleum ether part, chloroform part and water-soluble part. The chloroform part was subjected to silica gel column chromatography, followed by gradient elution with petroleum ether-acetone (20:1-5:5), and the fractions were collected. From petroleum ether-acetone 10:1 fraction, obtain turpentone B (2) 400mg; Petroleum ether-acetone 9:1 fraction is subjected to repeated column chromatography to obtain turpentone F (6) 710mg and Talentone H(8) 167mg; Petroleum ether-acetone 8:2 fractions were subjected to repeated column chromatography to obtain tylentinone C(3) 33mg, tungentone D(4) 220mg and tungentone G(7) 48 mg; The fraction of ...
Embodiment 2
[0054] Embodiment 2 Compounds of the present invention inhibit human tumor cell proliferation test in vitro
[0055] 1. Screening of SGC-7901, BGC-823, SMMC-7721, HCT cell proliferation
[0056] Using the modified MTT method, the tumor cells in logarithmic growth phase were added to a 96-well culture plate, 90 μL per well. Subsequently, 10 μL of test samples (0.31-20 μg / mL) in four concentrations were added to each well. Three replicate wells were set up for the sample addition group. cells at 37°C, 5% CO 2 After incubating in the incubator for 72 hours, add MTT solution 5 mg / mL, 10 μL. After continuing to cultivate for 4 hours, add triple solution [10% SDS-5% isobutanol-0.012mol / L HCl], 90 μL / well, and after 12 hours, measure the OD value of each well at a wavelength of 550 nm with a microplate reader.
[0057] Cell inhibition rate=(OD value of control group-OD value of medication group) / OD value of control group×100%. Calculate the IC of each sample with GWBASIC softwar...
Embodiment 3
[0061] Effects of Compounds of the Present Invention on Angiogenesis of Chicken Embryo Chorioallantoic Membrane (CAM)
[0062] experimental method:
[0063] (1) Put the eggs into the incubator at 37°C after being sterilized, with the air chamber upwards, and rotate 3-4 times a day. On the ninth day of hatching, after disinfecting the surface of the eggs, make a small hole of 1-2mm on the top of the air chamber, at a distance from the fetal head. Mark a rectangular area of 1.0cm×1.5cm on the projected part of the eggshell in the first 1cm and between the two yolk veins, grind and cut through the eggshell, and gently scratch a small hole with a diameter of about 1mm on the eggshell membrane, and add a little Separation of the eggshell membrane on the edge of the small hole with sterile pure water, put a sterile microporous filter membrane carrier with a diameter of 6mm on the least blood vessel of the CAM, and then add large, medium and small doses of the compound to be tested...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com