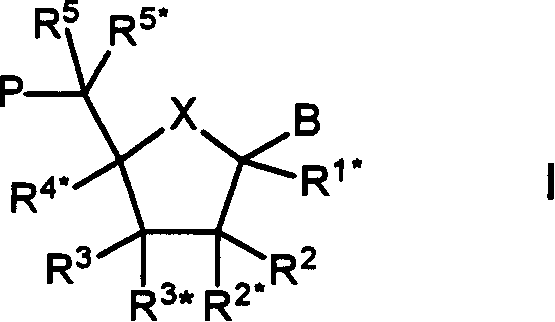
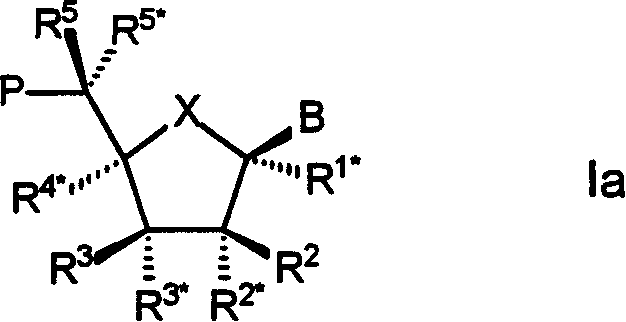
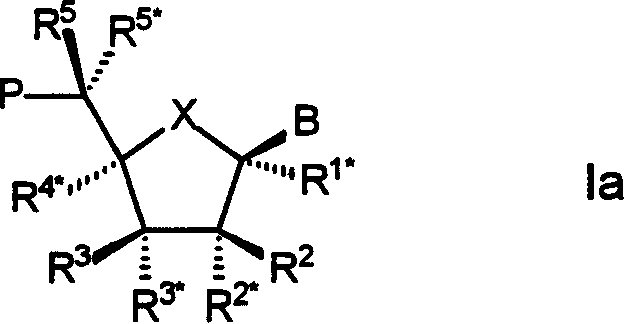
Bi-and tri-cyclic nucleoside, nucleotide and oligonucleotide analoguse
A technology of nucleoside analogs and analogs, which is applied in the field of bis- and tricyclic nucleoside analogs, and can solve problems such as hybridization specificity and complex methods, complex synthesis, and lack of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0291] 3-C-allyl-1,2-O-isopropylidene-α-D-ribofuranose (0A)
[0292] Method 1: 5-O-tert-butyldimethylsilyl-1,2-O-isopropylidene-α-D-nucleofuran-3-ketose (Y.Yoshimura, T.Sano , A.Matsuda and T.Ueda, Chem.Pharm.Bull., 1988,36,162) (17.8g, 58.9mmol) of anhydrous THF (980cm 3 ) solution and added dropwise in anhydrous ether (130cm 3 , 130 mmol) of 1M allylmagnesium bromide. After stirring for 2 hours, add a saturated aqueous solution of ammonium chloride (800cm 3 ) and dichloromethane (3×400cm 3 ) to extract the mixture. With salt water (3×450cm 3 ) to wash the organic phase and dry (sodium sulfate). The solvent was removed under reduced pressure and the residue was dissolved in anhydrous THF (700 cm 3 )middle. Add 1.1M tetrabutylammonium fluoride in THF (54.4cm 3 , 59.8 mmol) solution, the mixture was stirred at room temperature for 1 hour and evaporated to dryness. The residue was dissolved in dichloromethane (1700cm 3 ) and saturated aqueous solution of sodium bicarb...
Embodiment 2
[0295] 3-C-allyl-3,5-di-O-benzyl-1,2-O-isopropylidene-α-D-ribofuranose (0B)
[0296] Sodium hydride (4.9g, 123mmol) in anhydrous DMF (100cm 3 ) and added dropwise furanose OA (9.42g, 40.9mmol) in anhydrous DMF (65cm 3 ) solution. The solution was stirred at 50°C for 1 hour and cooled to 0°C. Benzyl bromide (14.5cm 3 , 121mmol) and anhydrous DMF (14.5cm 3 ) and stirred at room temperature for 18 hours. The reaction mixture was evaporated to dryness, washed with saturated aqueous sodium bicarbonate (2×450cm 3 ) Dichloromethane (700cm 3 ) solution and dried (sodium sulfate). The solvent was removed under reduced pressure, and the residue was purified by silica gel column chromatography using petroleum ether / ethyl acetate (9:1, v / v) as eluent to obtain compound OB (14.5 g, 86%) as an oil. δ H (CDCl 3 )7.39-7.21 (10H, m, Bn), 5.92 (1H, m, 2′-H), 5.71 (1H, d, J3.8, 1-H), 5.17-5.09 (2H, m, 3′- h a , 3′-H b ), 4.67(2H, m, Bn), 4.60(1H, d, J12.2, Bn), 4.52(1H, d, J12.1, Bn...
Embodiment 3
[0298] 3-C-allyl-1,2-di-O-acetyl-3,5-di-O-benzyl-D-ribofuranose (OC)
[0299] Stirring ribofuranose OB (12.42g, 30.3mmol) in 80% acetic acid (150cm 3 ) solution in aqueous solution for 3 hours. The solvent was removed under reduced pressure, and ethanol (3×75cm 3 ), toluene (3×75cm 3 ) of anhydrous pyridine (2×75cm 3 ) was co-evaporated with the residue and redissolved in anhydrous pyridine (60cm 3 ). Add acetic anhydride (46cm 3 ) and the solution was stirred at room temperature for 48 hours. Add ice and water (300cm 3 ) mixture, with diazomethane (2×300cm 3 ) to extract the resulting mixture. Sodium bicarbonate saturated aqueous solution (3 × 200cm 3 ) to wash the combined organic phases and dry (sodium sulfate). The solvent was evaporated, and the residue was purified by silica gel column chromatography using petroleum ether / ethyl acetate (4:1, v / v) as eluent to obtain the oily anomeric mixture OC (β:α~2:1)( 13.3 g, 97%). δ C (CDCl 3 ) 169.7, 169.6 (C=O), 138...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com