Chinese medicine prepn containing arasaponin and its prepn process
A technology of Panax notoginseng saponins and ginsenosides is applied in the directions of medical preparations containing active ingredients, pharmaceutical formulations, drug delivery, etc., and can solve the problems of irritation at the injection site, inconvenient administration, and influence on drug acceptability, etc. Achieve pure white color, increase acceptability, and facilitate administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
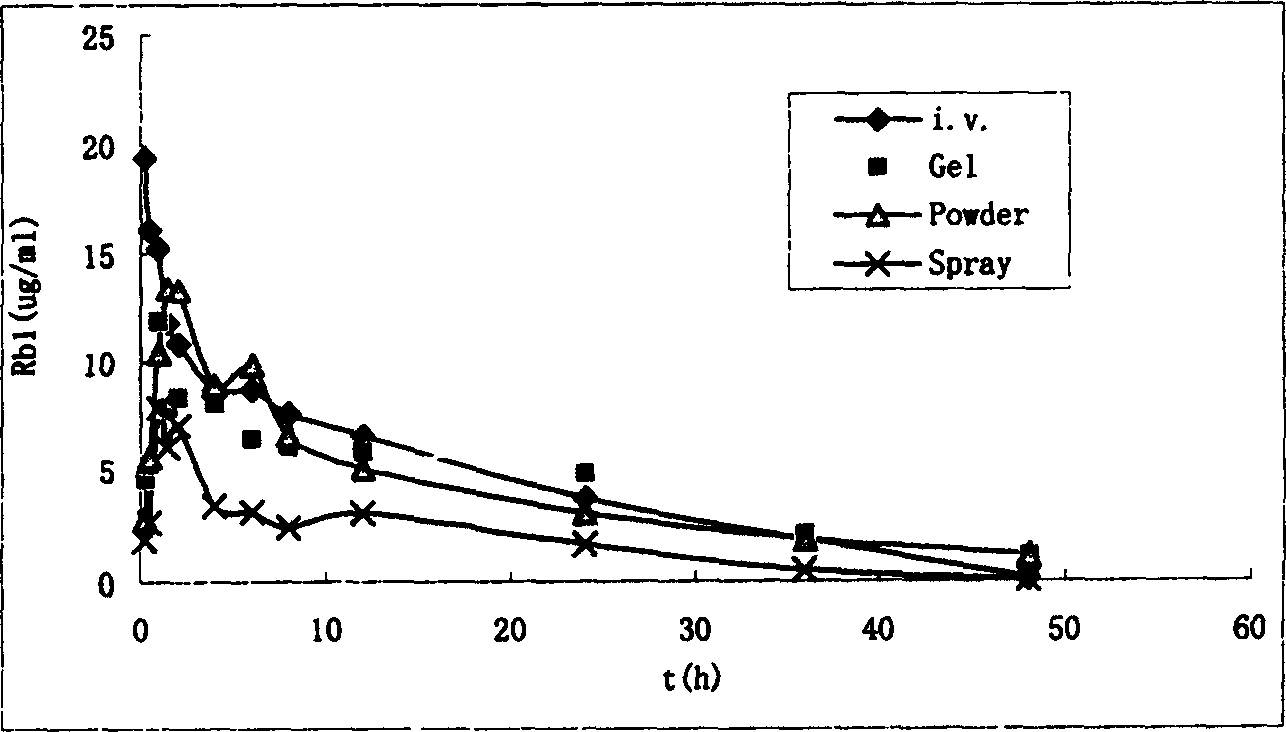
Image
Examples
Embodiment 1
[0038] Take 1g of Avicel PH-101 and 1g of PNS and place them in a mortar, add 20ml of distilled water, and grind in a grinder for 50min at a speed of 200rpm. Then freeze-dry in a freeze dryer for 18 hours, pass through a 200-mesh sieve, and obtain a dry powder spray.
Embodiment 2
[0040] Take 5g of Avicel PH-103 and 2g of PNS and place them in a mortar, add 100ml of distilled water, and grind in a grinder for 50min at a speed of 400rpm. Then freeze-dry in a freeze dryer for 24 hours, and pass through a 200-mesh sieve to obtain a dry powder spray.
Embodiment 3
[0042] Take 3g of calcium carbonate and 5g of PNS and place them in a mortar, add 50ml of distilled water, and grind in a grinder for 50min at a speed of 500rpm. Then freeze-dry in a freeze dryer for 22 hours, pass through a 220-mesh sieve to obtain a dry powder; take 1 g of hydroxypropyl cellulose and swell it in 100 ml of distilled water for 12 hours, then fully stir to disperse evenly and become a transparent gel matrix; Stir and mix the dry powder to obtain a gel spray with a PNS concentration of 50 mg / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com