Recombinant human alpha-prothymosin interleukin 2 gene and its expression and use
A technology of pro-thymosin and interleukin, applied in gene therapy, recombinant DNA technology, peptide/protein components, etc., can solve problems such as large toxic and side effects, achieve high biological activity, and overcome safety problems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 Thymocyte Total RNA Extraction and Reverse Transcription PCR
[0018] Take 3-4 month old fetal thymus, prepare single cells, suspend in RPMI1640 medium, cell density is 1×10 7 ml, under the combined stimulation of 20ug / ml PHA and 500U / ml IL-2 at 37°C, 5% CO 2 Cultivate under the conditions for 24 hours, take the cultured thymocytes and extract total RNA according to the method of TAKARA total RNA kit, dissolve in DEPC-treated deionized water, take a certain amount of RNA, add downstream primer (P2) and dNTP, in Reverse transcriptase was used for 30 minutes at 42°C to reverse transcribe α-prothymosin mRNA into single-stranded cDNA, and further add upstream primer (P1) and Taq enzyme for PCR to obtain α-prothymosin cDNA. The upstream and downstream primers are as follows: upstream primer 5'GTC AAG CTT ATG TCA GAC GCA GCC GTA G3'downstream primer 5'ACT GGA TCC TTA GTC ATC CTC GTC GGT CTT C3'
Embodiment 2I
[0019] Embodiment 2 IL-2 gene RT-PCR process
[0020] Take 5ml of heparin anticoagulant blood, use the density gradient method to separate peripheral blood PBMCs in the lymphocyte separation medium, and use RPMI1640 to adjust the number of cells to (2~3)×10 6 / ml, add PHA10ug, interleukin-250IU respectively, at 37°C, 5% CO 2 Culture under conditions for 24 to 48 hours; collect the above cells, centrifuge at 1000r / min for 5 minutes, take the bottom cells and wash them with PBS buffer, then extract the total RNA of the cells with guanidine phenol chloroform isothiocyanate in one step. The obtained RNA precipitate was dissolved in diethyl pyrocarbonate (DECP) treated water, identified by glyoxal 1% agarose gel electrophoresis (containing 0.5 μg of ethidium bromide), and the total RNA concentration was measured by a UV spectrophotometer. OD260nm / 280nm between 1.8 and 2.0, store in -20°C refrigerator; follow the instructions of TAKARA's reverse transcription kit, use the extracted...
Embodiment 3
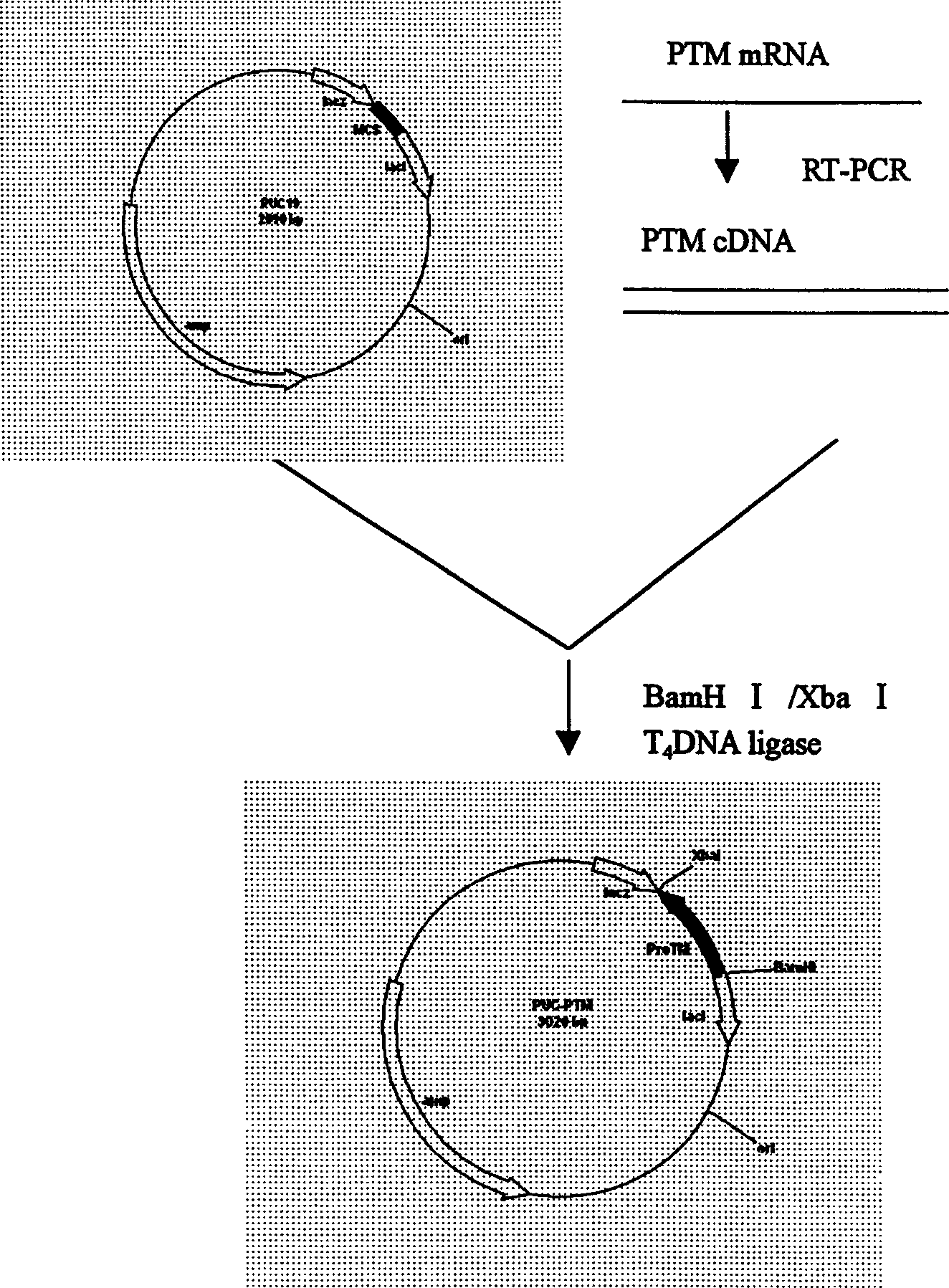
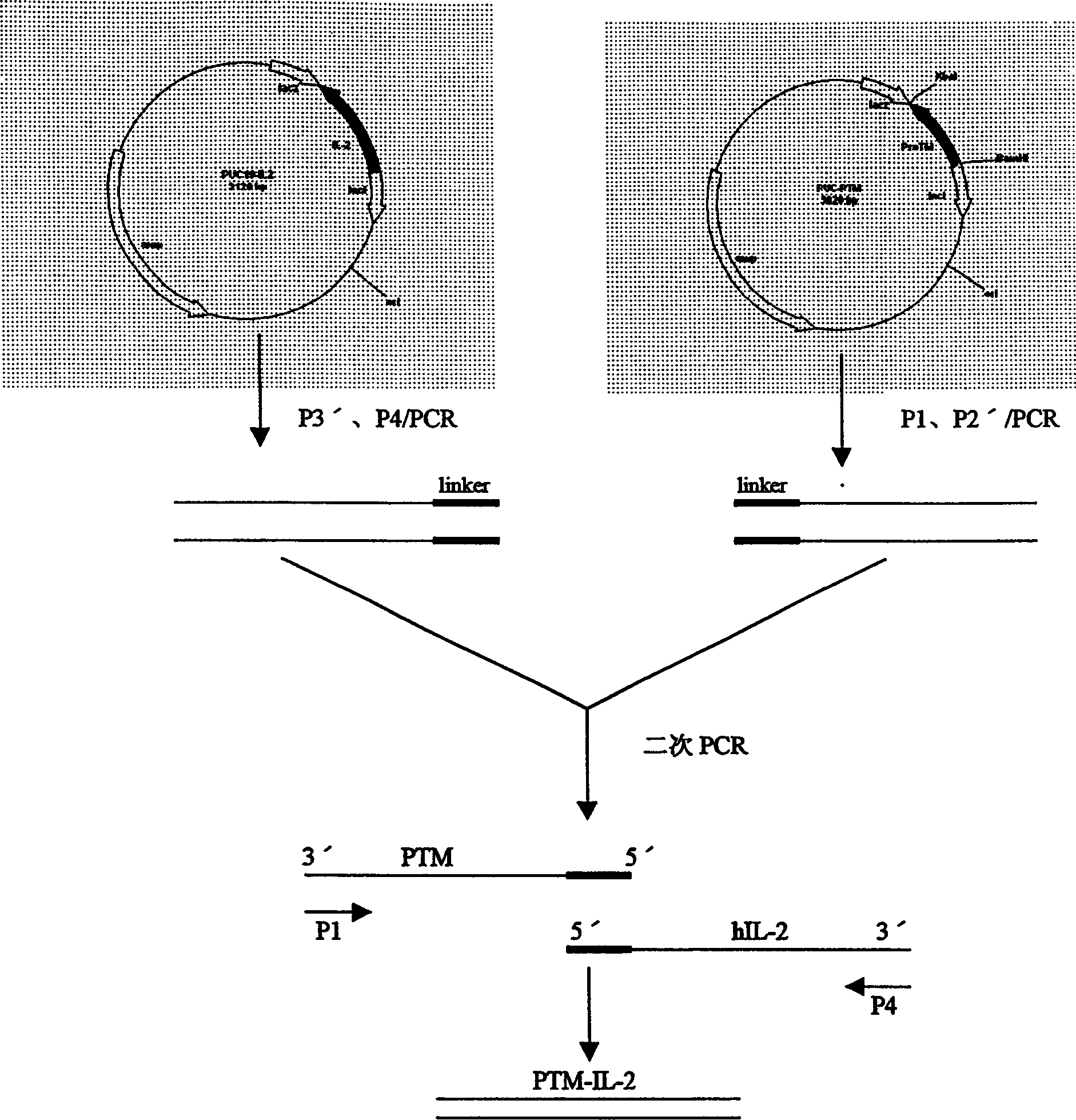
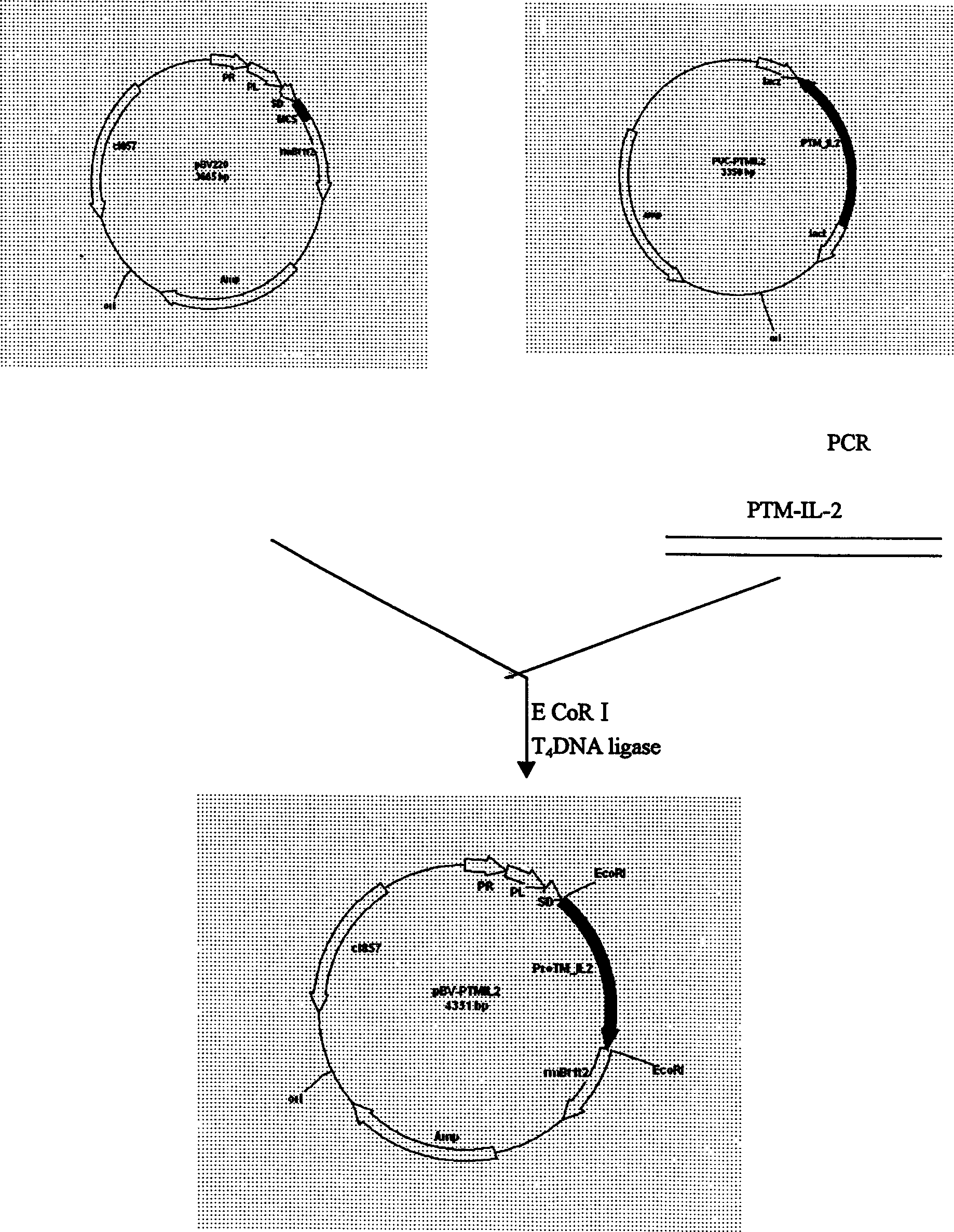
[0023] Example 3 Human α-prothymosin functional region gene cloning
[0024] Use bio-soft, sequence and other biological software to determine the optimal annealing temperature through computer analysis, avoiding the secondary structure of mRNA that may interfere with the initiation of translation; ensure that there is no specific sequence pairing between the two primers, and that there is specific complementarity between the primer templates Pairing to avoid amplification of unnecessary sequences; the 5' of the upstream and downstream primers described in Example 1 were respectively introduced into BamHI and XbaI restriction sites, and artificially synthesized oligonucleotide primers: human α-prothymosin cDNA As a template, the human α-prothymosin gene fragment was obtained after PCR amplification. After the above fragment was treated with BamHI / XbaI restriction endonuclease, the protein was extracted by phenol / chloroform, and the DNA was extracted by ethanol precipitation, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com