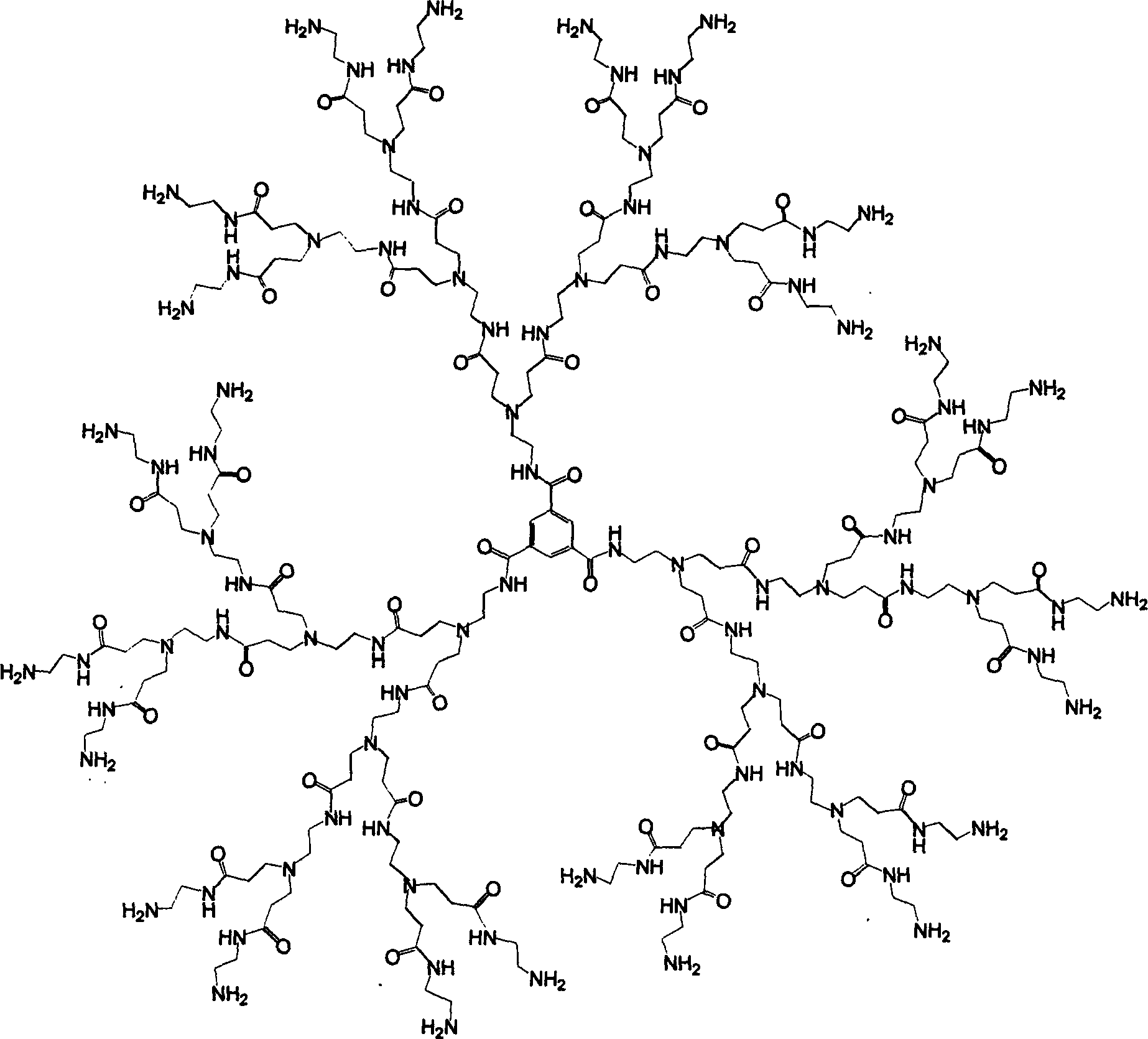
Tree-type high-molecular polyamide-amine compound and its preparing process and application
A polymer and polyamide technology, applied in the direction of using vectors to introduce foreign genetic material, recombinant DNA technology, etc., can solve the problems of poor biodegradability and high toxicity, achieve enhanced targeting, reduce cytotoxicity, and improve transfection efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1. Preparation of trimethyl isophthalic acid G0.5: 2.8 grams of isophthalic acid crystals (melting point 376° C.) recrystallized from water were added to 26 ml of anhydrous methanol, 0.5 ml of concentrated H 2 SO 4 , Refluxed for 2 hours, and 2 / 3 of the methanol was evaporated. Distilled methanol with anhydrous K 2 CO 3 dry. The dried methanol was injected back into the reaction flask and refluxed for 2 hours. Repeat this three times. When the reaction mixture was cooled, a large number of white crystals appeared. The filtered crystals were dissolved in 30ml of ether, filtered, the filtrate was poured into 30ml of water, and then extracted with 105ml of ether. The ether solutions were combined with 100 ml of 10% KHCO 3 The solution was thoroughly washed 2 times, and then washed 2 times with water, and the separated ether layer was washed with anhydrous MgSO 4 dry. The dried ether solution was filtered, and the filtrate was sucked to dry ether under redu...
Embodiment 2
[0101] Example 2. Preparation of the above-mentioned G1.0: Dissolve 1.4 grams of G0.5 in 70ml of anhydrous methanol with slight heating, place it in a dropping funnel, and slowly drop into 75ml of it in an ice bath, nitrogen protection, and electromagnetic stirring In anhydrous ethylenediamine, stir at room temperature for 72 hours in the dark. The methanol and a small amount of ethylenediamine were evaporated under reduced pressure with a water pump at 30°C, and then the excess ethylamine was evaporated under reduced pressure at 30°C and a vacuum of 1 mmHg. The obtained yellow oil was dissolved in 10ml of anhydrous methanol, and then 100ml of anhydrous toluene was added, and methanol and toluene were distilled off under reduced pressure. Repeat this three times to obtain 1.6 grams of white powder which is the G1.0 product. Yield 85.6%.
Embodiment 3
[0102] Example 3. Preparation of the above-mentioned semi-generation product: place the methanol solution of the whole-generation product in a three-necked round-bottomed flask, and slowly drip it into the periphery of the whole-generation product with a syringe under ice bath, nitrogen protection, and electromagnetic stirring. Methyl acrylate with 2.4 times the molar number of amine groups, and then a catalytic amount of sodium methoxide in methanol was dripped into the solution with a syringe, and stirred at room temperature for 72 hours in the dark. Evaporate methanol and excess methyl acrylate under reduced pressure with a water pump at 30°C, dissolve with a small amount of methanol, add ether, and decant the supernatant. Repeat several times, and dry under reduced pressure to obtain a yellow viscous substance which is the desired half-generation polyamide-amine dendrimer. The yield is about 94%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com