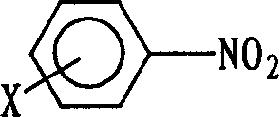
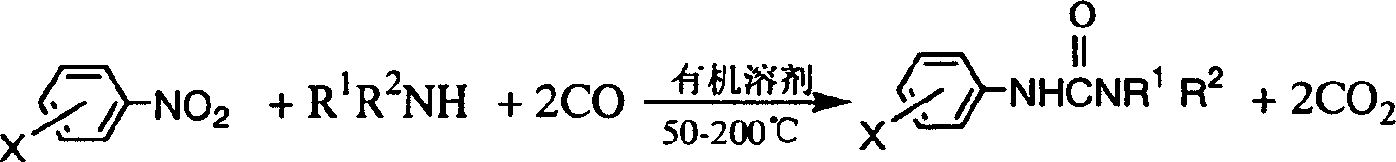Method for synthesizing compound of asymmetric substituted carbamide class from carbonylation
A chemical synthesis and asymmetric technology, applied in the preparation of organic compounds, chemical instruments and methods, preparation of urea derivatives, etc., can solve the problems of high price, achieve stable product quality, facilitate large-scale industrial production, and select reactions sex high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In a 70mL stainless steel autoclave, add p-chloronitrobenzene (10mmol), Se (0.5mmol), 33% dimethylamine aqueous solution (20mmol), Et 3 N (1ml) and acetone (10ml), replaced by CO three times, raised the pressure of CO to 3MPa, put it into an oil bath that had risen to 135°C, stirred and reacted for 4h, cooled to room temperature, opened the kettle, and filtered the obtained The solid and the mother liquor were concentrated and the solid obtained by filtration was combined, recrystallized, dried, and weighed to obtain 1.0812g product N, N-dimethyl-N'-p-chlorophenylurea, and the HPLC analysis purity was more than 99.6%. It is 54.5% (in p-chloronitrobenzene) for the first single-pass yield, and the determination of content adopts Waters high-performance liquid chromatography system, including two 515 pumps, 486 type UV detectors, Spherisorb ODS-2 post (5 μ m, 4.6 × 250mm), with methanol-water as mobile phase, flow rate: 1mL / min, detector wavelength is λmax of each compound...
Embodiment 2
[0025] The organic solvent is benzene, and the experimental method and steps are the same as in Example 1. The HPLC analysis purity is more than 99%, and the actual first single-pass yield is 32.4% (in p-chloronitrobenzene).
Embodiment 3
[0027] The organic solvent is n-hexane, and the experimental method and steps are the same as in Example 1. The HPLC analysis purity is more than 99%, and the actual first-time single-pass yield is 35.6% (in terms of p-chloronitrobenzene).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com