Fluorescent probe based on 1, 8-naphthalimide derivative as well as preparation method and application of fluorescent probe
A fluorescent probe, naphthalimide technology, which is applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of cumbersome preparation methods of fluorescent probes, low yield, and inability to identify hydrogen sulfide with naked eyes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
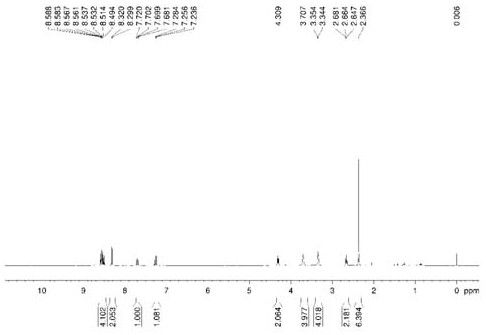
Image
Examples
Embodiment 1
[0044] This embodiment is a preparation method of a fluorescent probe SBD based on 1,8-naphthalimide derivatives, and the steps are as follows:
[0045] (1) (Intermediate 1) Preparation of 6-bromo-2-(2-(dimethylamino)ethyl)-1H-benzo[d,e]isoquinoline-1,3(2H)-dione :
[0046] In a 50 mL single-neck flask, commercial 4-bromo-1,8-naphthalene anhydride (276 mg, 1 mmol) and dimethyldiamine (163 μL, 1.5 mmol) were dissolved in ethanol (25 mL), and the reaction was stirred under reflux conditions. After cooling to room temperature for 5 h, a large amount of solid was precipitated, which was filtered by suction and washed with ice ethanol to obtain a light yellow solid, which was Intermediate 1 (173 mg), and the yield was 50%;
[0047] (2) (Intermediate 2) 2-(2-(Dimethylamino)ethyl)-6-(piperazin-1-yl)-1H-benzisoquinoline-1,3(2H)-dione Preparation of:
[0048] In a 50 mL single-necked flask, intermediate 1 (346 mg, 1 mmol) and piperazine (235 μL, 3 mmol) were dissolved in ethylene gl...
Embodiment 2
[0054] This embodiment is a preparation method of a fluorescent probe SBD based on 1,8-naphthalimide derivatives, and the steps are as follows:
[0055] (1) (Intermediate 1) Preparation of 6-bromo-2-(2-(dimethylamino)ethyl)-1H-benzo[d,e]isoquinoline-1,3(2H)-dione :
[0056] In a 50 mL single-neck flask, commercial 4-bromo-1,8-naphthalene anhydride (276 mg, 1 mmol) and dimethyldiamine (326 μL, 3 mmol) were dissolved in ethanol (25 mL), and the reaction was stirred for 6 h under reflux conditions. , after cooling to room temperature, a large amount of solid was precipitated. After suction filtration and washing with ice ethanol, a pale yellow solid was obtained, which was Intermediate 1 (225 mg), and the yield was 65%;
[0057] (2) (Intermediate 2) 2-(2-(Dimethylamino)ethyl)-6-(piperazin-1-yl)-1H-benzisoquinoline-1,3(2H)-dione Preparation of:
[0058] In a 50 mL single-necked flask, intermediate 1 (346 mg, 1 mmol) and piperazine (470 μL, 6 mmol) were dissolved in ethylene gly...
Embodiment 3
[0064] This embodiment is a preparation method of a fluorescent probe SBD based on 1,8-naphthalimide derivatives, and the steps are as follows:
[0065] (1) (Intermediate 1) Preparation of 6-bromo-2-(2-(dimethylamino)ethyl)-1H-benzo[d,e]isoquinoline-1,3(2H)-dione :
[0066] In a 50 mL single-necked flask, commercial 4-bromo-1,8-naphthalene anhydride (276 mg, 1 mmol) and dimethyldiamine (435 μL, 4 mmol) were dissolved in ethanol (25 mL), and the reaction was stirred for 10 h under reflux conditions. , after cooling to room temperature, a large amount of solid was precipitated. After suction filtration and washing with ice ethanol, a pale yellow solid was obtained, which was Intermediate 1 (277 mg), and the yield was 80%;
[0067] (2) 2-(2-(Dimethylamino)ethyl)-6-(piperazin-1-yl)-1H-benzisoquinoline-1,3(2H)-dione (Intermediate 1) Preparation of:
[0068] In a 50 mL single-necked flask, intermediate 1 (346 mg, 1 mmol) and piperazine (775 μL, 10 mmol) were dissolved in ethylene g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com