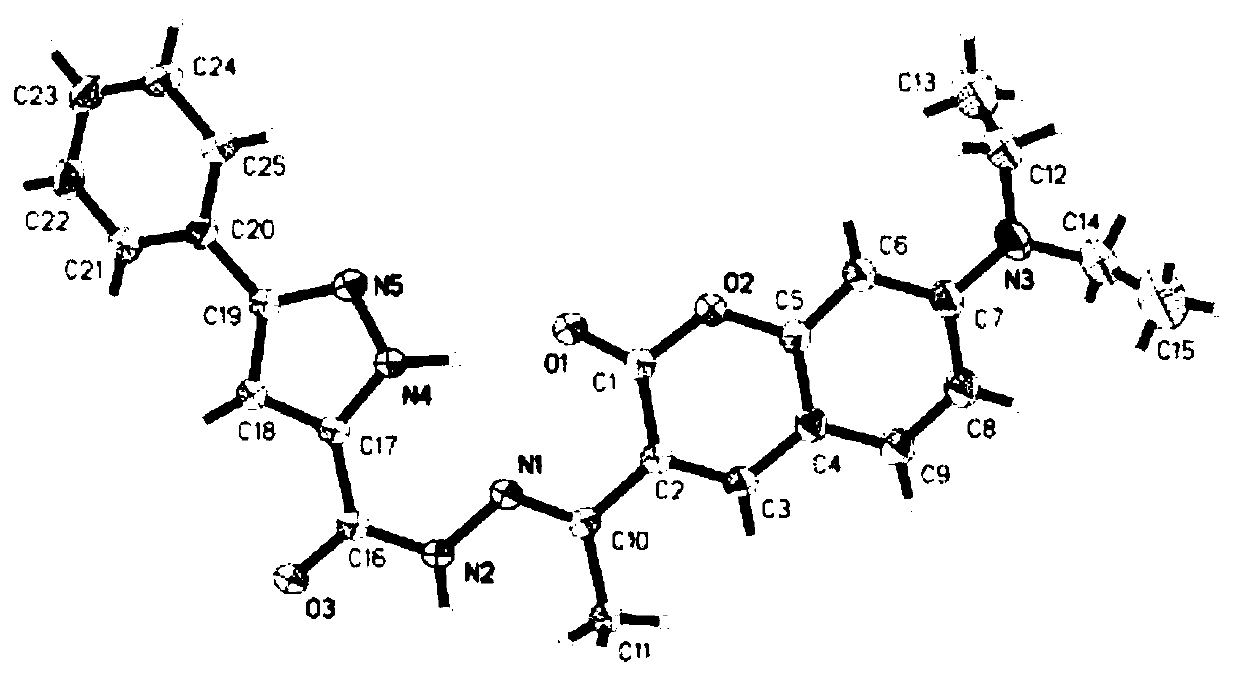
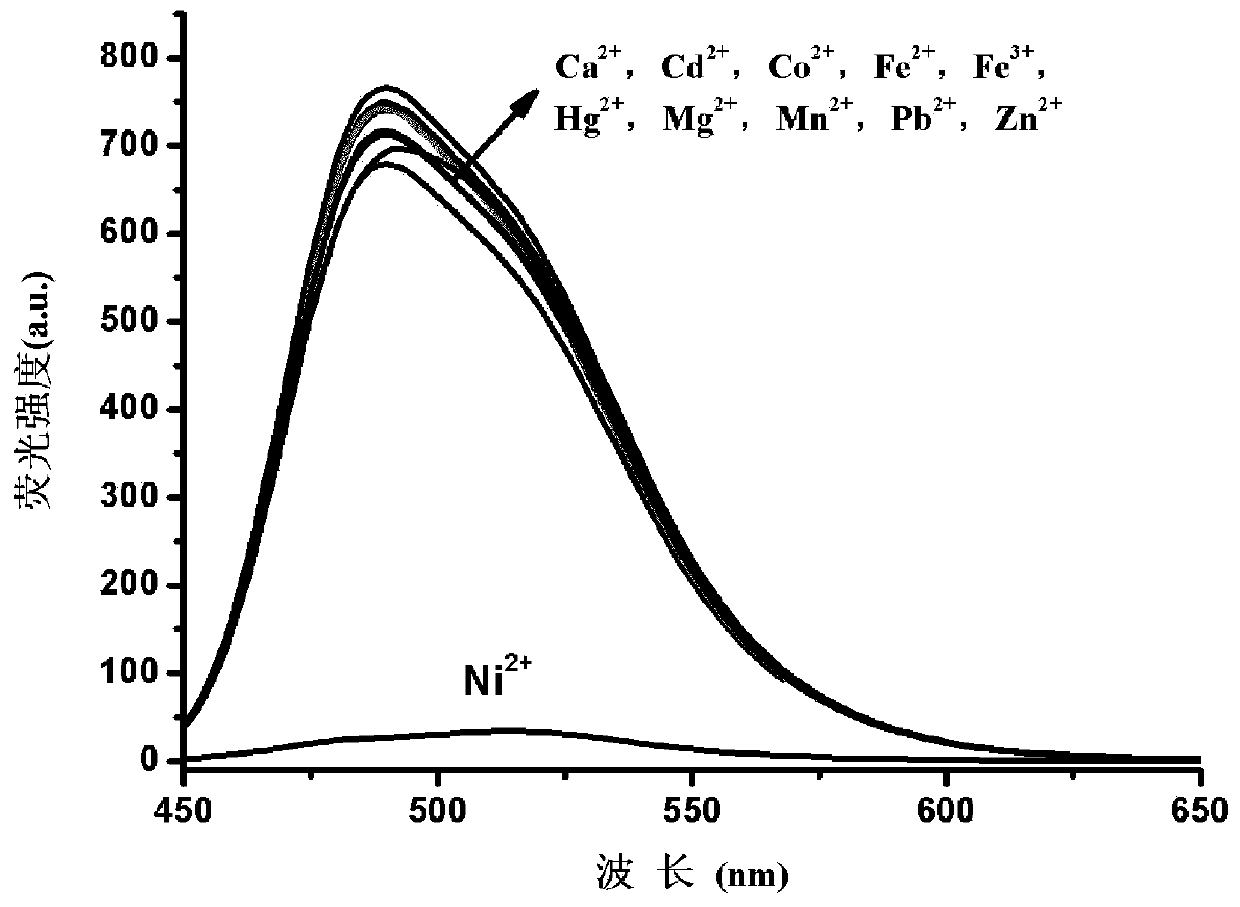
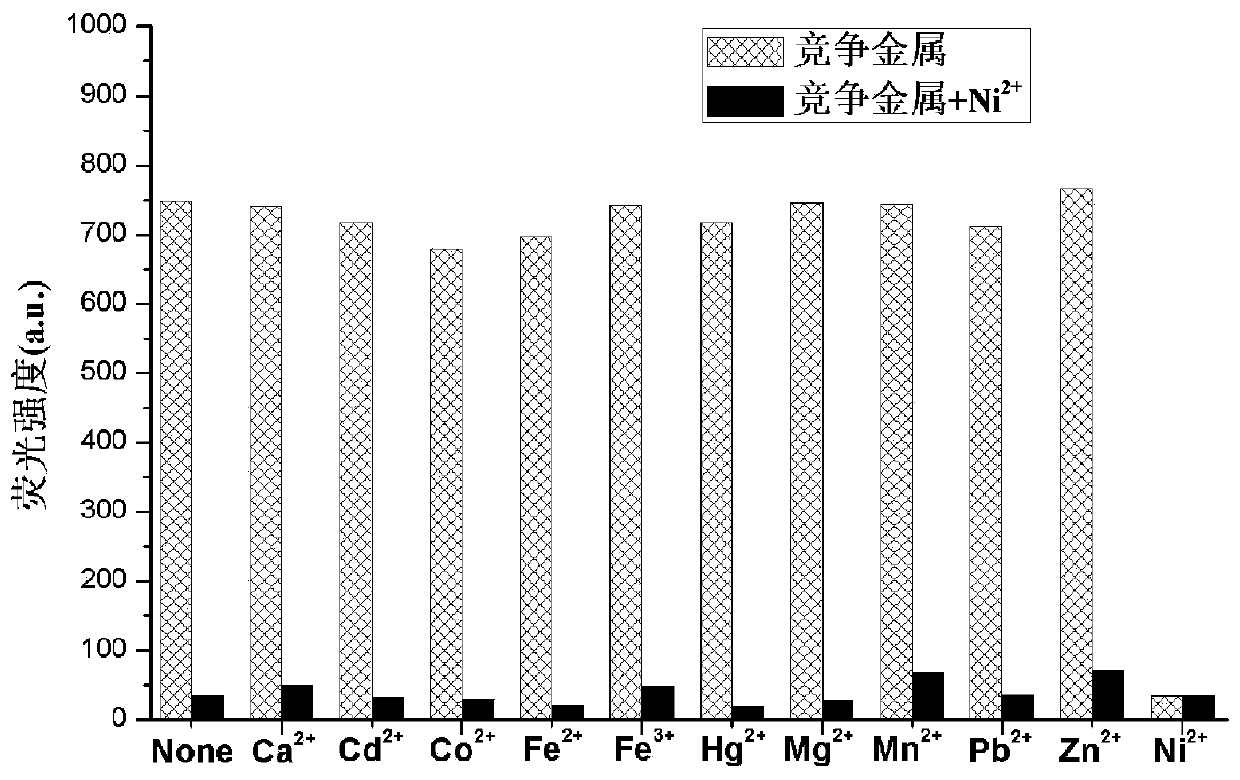
A kind of coumarin nickel ion fluorescent probe and its preparation method, crystal preparation method and application
A fluorescent probe, coumarin-based technology, applied in fluorescence/phosphorescence, organic chemistry methods, chemical instruments and methods, etc., can solve the problems of poor anti-interference ability, low sensitivity, long response time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: This embodiment provides the preparation method of preparing required hydrazide intermediate, and its reaction equation is as follows:
[0032]
[0033] Specific steps are as follows:
[0034] Add methyl phenylpyrazolylcarboxylate (2.02g, 0.01mol), 20mL methanol, and 5mL 80% hydrazine hydrate into a 50mL round-bottomed flask, reflux the mixed solution in an oil bath at 80-90°C for 2h, and cool to room temperature , the solvent was removed from the resulting mixture under reduced pressure, and the filter residue was washed with water to obtain a white solid, which was the hydrazide intermediate, with a yield of 93%.
Embodiment 2
[0035] Embodiment 2: This embodiment provides the synthesis method of phenylpyrazole acylhydrazone diethylaminocoumarin fluorescent probe, and its reaction equation is as follows:
[0036]
[0037] In a 50mL round-bottomed flask, add the hydrazide intermediate (1.01g, 0.005mol) prepared in Example 1, 3-acetyl-7-(diethylamino)coumarin (1.30g, 0.005mol), 2 Drop formic acid, then add 10 mL of absolute ethanol, reflux the mixed solution in an oil bath at 80-90°C for 3 hours, cool to room temperature, filter the filter residue under reduced pressure, and wash the obtained solid with absolute ethanol and ether in sequence to obtain a yellow powder. Phenylpyrazolehydrazone-based diethylaminocoumarin fluorescent probe with a yield of 86%.
[0038] M.p.190-192℃; IR (KBr, pellet, cm-1): ν3419(br), 1710(s), 1665(s), 1622(s); 1H NMR(500MHz, CDCl3, ppm): 8.10(s , 1H, Aryl-H), 7.88-7.81 (m, 4H, Aryl-H), 7.55-7.39 (m, 3H, Aryl-H), 6.97 (d, J=7.4Hz, 1H, Aryl-H), 6.55 (s, 1H, CH-pyrazole)...
Embodiment 3
[0039] Embodiment 3: This embodiment provides the synthesis method of phenylpyrazole acylhydrazone diethylaminocoumarin fluorescent probe:
[0040] In a 50mL round-bottomed flask, add the hydrazide intermediate (1.01g, 0.005mol) prepared in Example 1, 3-acetyl-7-(diethylamino)coumarin (1.30g, 0.005mol), 2 Drop formic acid, then add 10 mL of absolute ethanol, reflux the mixed solution in an oil bath at 80-90°C for 5 hours, cool to room temperature, filter the filter residue under reduced pressure, and wash the obtained solid with absolute ethanol and ether in sequence to obtain a yellow powder. Phenylpyrazolehydrazone-based diethylaminocoumarin fluorescent probe with a yield of 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com