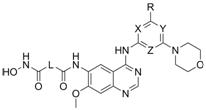
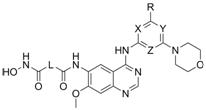
4-aminoquinazoline compound containing morpholinyl aromatic heterocycle and hydroximic acid structure and preparation method thereof
A technology of aminoquinazoline and compounds, which is applied in the field of synthesis of 4-aminoquinazoline compounds, can solve the problems of drug resistance and other problems, and achieve the effects of high yield, mild reaction conditions, and safe and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
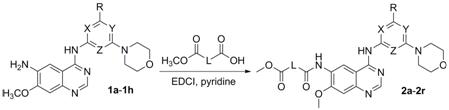
[0050] Example 1: Methyl 4-((4-((4-morpholinylpyridin-2-yl)amino)-7-methoxyquinazolin-6-yl)amino)-4-oxobutyrate Preparation of (2a)
[0051] Add N to a single-neck bottle 4 -(4-Morpholinylpyridin-2-yl)-7-methoxyquinazoline-4,6-diamine (1a) 0.8 mmol, 12 mL of pyridine, after stirring to dissolve, 2 mmol of monomethyl succinate was added , 1-ethyl-3-(3-dimethylpropylamine) carbodiimide hydrochloride (EDCI) 3mmol, reacted at room temperature for 6h, distilled under reduced pressure to dryness, added water, suction filtered, washed with saturated sodium bicarbonate, washed with water , or washed with water, placed at room temperature with water, filtered with suction, washed with saturated sodium bicarbonate, washed with water, and dried under vacuum at 40 °C for 24 h to obtain a light yellow solid 4-((4-((4-morpholinopyridin-2-yl)amino) -7-Methoxyquinazolin-6-yl)amino)-4-oxobutyric acid methyl ester (2a) 0.31g, yield 83.1%;
[0052] 1 HNMR (400MHz, DMSO-d 6 ), δ: 9.77(s, 1H)...
Embodiment 2
[0053] Example 2: Methyl 5-((4-((4-morpholinylpyridin-2-yl)amino)-7-methoxyquinazolin-6-yl)amino)-5-oxopentanoate Preparation of (2b)
[0054] According to the preparation method of Example 1, succinate monomethyl ester was replaced with glutaric acid monomethyl ester to obtain yellow solid 5-((4-((4-morpholinopyridin-2-yl)amino)-7 -Methoxyquinazolin-6-yl)amino)-5-oxopentanoate methyl ester (2b) 0.34 g, yield 88.4%.
[0055] 1 HNMR (400MHz, DMSO-d6), δ: 9.77(s, 1H), 9.26(s, 1H), 8.98(s, 1H), 8.80(s, 1H), 7.97(d, J=7.5Hz, 1H) , 7.29(s, 1H), 6.24(dd, J=7.4,1.5Hz, 1H), 6.01(d, J=1.5Hz, 1H), 4.01(s, 3H), 3.75(t, J=7.1Hz, 4H), 3.62(s, 3H), 3.39(t, J=7.1Hz, 4H), 2.46(dt, J=10.1,7.1Hz, 4H), 1.97-1.92(m, 2H); 13 CNMR(100MHz,DMSO-d6),δ:173.13, 171.89, 155.85, 154.84, 154.48, 154.13, 151.30, 147.49, 147.39,128.64, 114.14, 112.01, 104.32, 97.96, 97.36, 66.62(2C), 56.14, 51.38, 48.31(2C), 36.29, 33.01, 21.57.
Embodiment 3
[0056] Example 3: Methyl 6-((4-((4-morpholinylpyridin-2-yl)amino)-7-methoxyquinazolin-6-yl)amino)-6-oxohexanoate Preparation of (2c)
[0057] According to the preparation method of Example 1, monomethyl succinate was replaced with monomethyl adipate to obtain yellow solid 6-((4-((4-morpholinopyridin-2-yl)amino)-7 -Methoxyquinazolin-6-yl)amino)-6-oxohexanoic acid methyl ester (2c) 0.31 g, yield 78.3%.
[0058] 1 HNMR (400MHz, DMSO-d6), δ: 9.77(s, 1H), 9.27(s, 1H), 8.97(s, 1H), 8.79(s, 1H), 7.97(d, J=7.5Hz, 1H) , 7.30(s, 1H), 6.24(dd, J=7.4,1.5Hz, 1H), 6.01(d, J=1.5 Hz, 1H), 4.01(s, 3H), 3.75(t, J=7.1Hz, 4H), 3.62(s, 3H), 3.39(t, J=7.1Hz, 4H), 2.34(td, J=7.0,3.0Hz, 4H), 1.71-1.67(m, 2H), 1.61-1.56(m , 2H); 13 CNMR (100MHz, DMSO-D6), Δ: 173.68, 171.80, 155.86, 154.88, 154.46, 154.13,151.17, 147.85, 147.26, 128.63, 112.07, 104.22, 97.67, 56.14, 56.14, 56.14, 56.3. 48.31(2C), 36.70, 33.60, 24.09, 24.02.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com