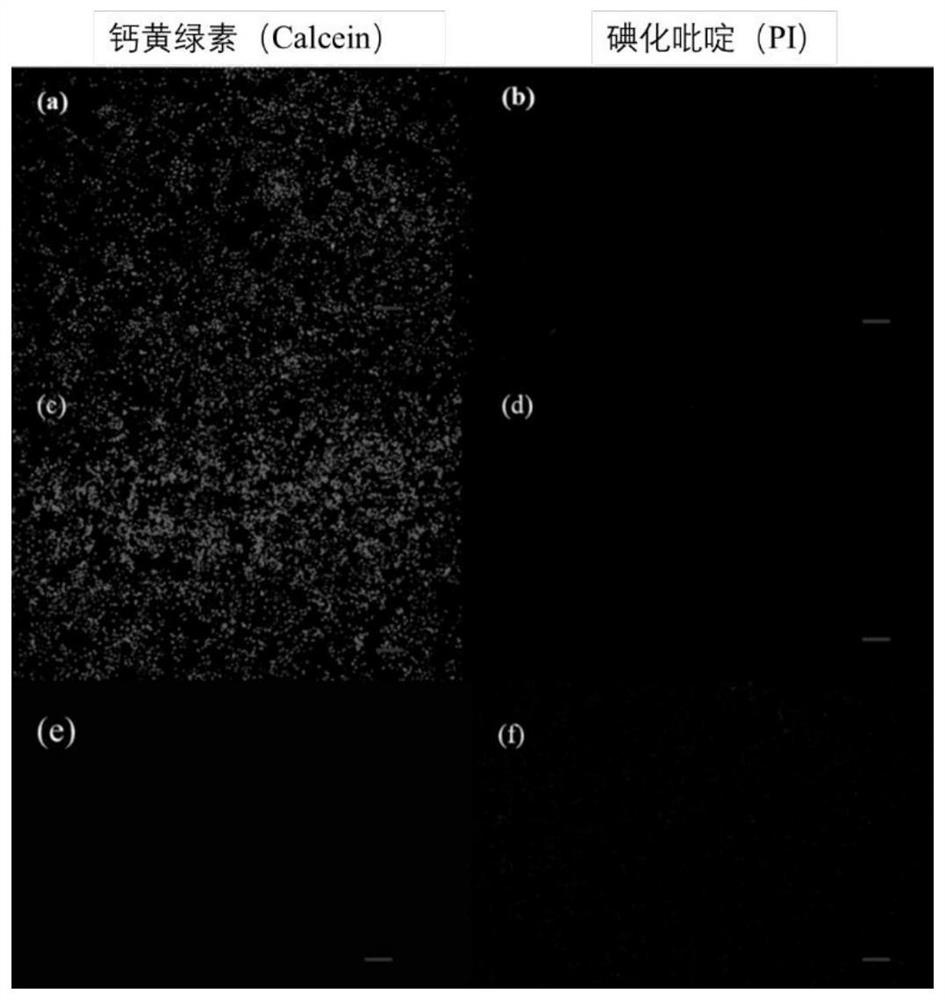
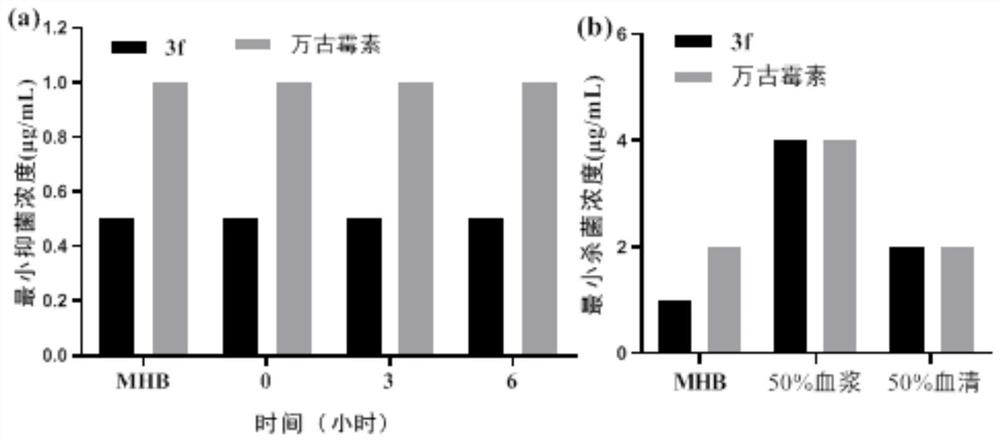
Tri-cation quaternary ammonium salt antibacterial peptide simulant with antibacterial activity and preparation method thereof
A technology of triple cations and quaternary ammonium salts, which is applied in the preparation of organic compounds, aminohydroxy compounds, antibacterial drugs, etc., and can solve the problems of high cytotoxicity of host cells, high production costs, and large molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Preparation of Example 1 Compound 1a:
[0072] In a round-bottom flask (100 mL), phloroglucinol (7.93 mmol, 1 eq.) was dissolved in acetone (20 mL), then solid potassium carbonate (35.68 mmol, 4.5 eq.) was added, and 3-bromo was added after stirring evenly. -1-Propanol (35.68mmol, 4.5eq.), heated to reflux at 65°C, and reacted for 24h. After the reaction, the solvent was concentrated and removed, ethyl acetate (100 mL) and water (100 mL) were added to dissolve and dilute the solid, the organic phase was washed three times with water (100 mL), the organic phase was retained and washed with saturated sodium chloride solution (10 mL, 1- 3 times) and dried over anhydrous sodium sulfate, filtered after drying, and the organic phase was concentrated. Weigh the concentrated product (3 g, 1 eq.), transfer it to a clean reactor, add dichloromethane (50 mL) to dissolve, and add phosphine tribromide (19.98 mmol, 2 eq.) dropwise to the system under an ice-water bath. , after the ...
Embodiment 2
[0074] Preparation of Example 2 Compound 1b:
[0075] In a round bottom flask (100 mL), dissolve phloroglucinol (7.93 mmol, 1 eq.) in N,N-dimethylformamide (20 mL), then add potassium carbonate solid (35.68 mmol, 4.5 eq.) , After stirring evenly, dibromobutane (35.68mmol, 4.5eq.) was added, and the reaction was carried out at room temperature for 24h. After the reaction, ethyl acetate (100 mL) and water (100 mL) were added to dilute the system, extracted, and the organic phase was retained. The organic phase was washed 3-5 times with water (100 mL), and washed with saturated sodium chloride solution (10 3 times) and anhydrous sodium sulfate to dry the organic phase, filter and concentrate the organic phase, and use column separation to purify the product (petroleum ether:ethyl acetate=20:1, V:V).
[0076] 1b: Yield 49%. 1 H NMR (400MHz, Chloroform-d) δ6.05(s, 3H), 3.95(t, J=6.0Hz, 6H), 3.48(t, J=6.6Hz, 6H), 2.10–2.01(m, 6H) ,1.93(m,6H). 13 C NMR (101MHz, Chloroform-d) δ160...
Embodiment 3
[0077] Example 3 Preparation of compound 1c: The preparation method was the same as that of Example 2, and the dibromoalkane used was dibromopentane.
[0078] 1c: Yield 47%. 1 H NMR (400MHz, Chloroform-d) δ6.05(s, 3H), 3.92(t, J=6.3Hz, 6H), 3.43(t, J=6.8Hz, 6H), 1.98–1.88(m, 6H) ,1.84–1.74(m,6H),1.66–1.57(m,6H). 13 CNMR (101MHz, Chloroform-d) δ160.81, 93.88, 67.59, 33.61, 32.47, 28.39, 24.86.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com