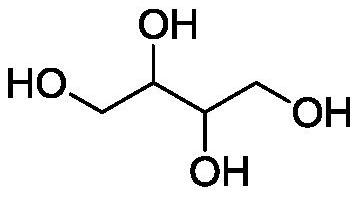
Method for preparing erythritol by electrochemical method
A technology of erythritol and electrolyzer, which is applied in the field of electrochemistry, can solve the problem of low atom economy and achieve the effect of good atom economy and high atom utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
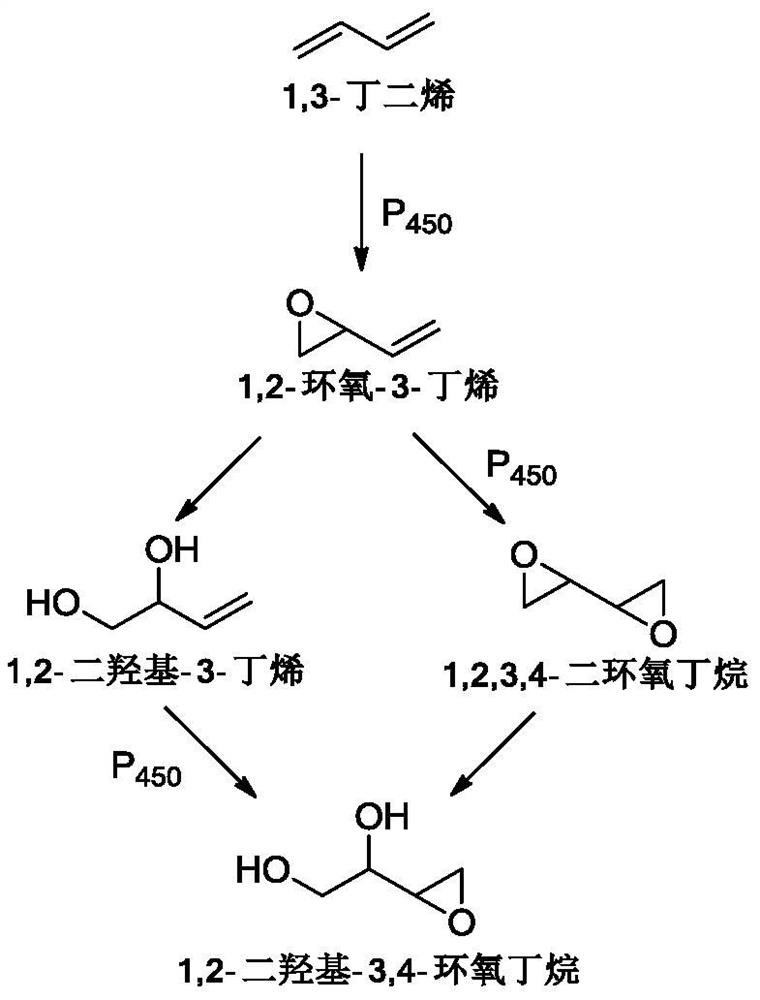
[0083] One-pot preparation of erythritol using meshed Ti / Pt as anode zero-pole-distance electrolyzer.
[0084] The zero-pole-distance electrolytic cell is used, and the cathode is selected as a mesh 316L stainless steel electrode, and the effective area of the anode and cathode is 75cm. 2 , Nafion 427 was selected as the cation exchange membrane.
[0085] Take 100g of acetonitrile, 400mL of water and 12g of sodium bromide into the anolyte storage tank and the catholyte storage tank respectively, adjust the mass flow of 1,3-butadiene to 0.065g / min, the current to 7.5A, and the current density to 1000A / m 2 , the current efficiency is 90%, the voltage is 3.6V, and the anolyte is continuously entered into the cathode liquid storage tank with a mass flow of 0.11g / min using a peristaltic pump for alkaline hydrolysis and saponification into a ring. After the catholyte reaction solution was transferred out, the aqueous solution of 1,2,3,4-diepoxybutane was alkaline and the pH value...
Embodiment 2
[0087] Erythritol was prepared by a one-pot method in a zero-pole-distance electrolytic cell by changing the ratio of acetonitrile / water, using potassium bromide halide as electrolyte and meshed Ti / Pt as anode.
[0088] A zero-pole-distance electrolytic cell was used, and the acetonitrile / water ratio was adjusted to be 1:2, that is, 167 g of acetonitrile, 333 g of water and 10 g of potassium bromide were used as the reaction solution, Nafion 115 was selected as the cation exchange membrane, and other conditions were as in Example 1.
[0089] After 1,2,3,4-diepoxybutane is synthesized by paired electrolysis, the reaction solution undergoes acid dissociation to open the ring, freeze crystallization, and dry to obtain white crystal erythritol. The electrolysis current and current density remain unchanged, and the current The efficiency is 87%, the voltage is 4.1V, the total yield is 80%, and the electrolysis energy consumption is 5874kWh / t.
Embodiment 3
[0091] Erythritol was prepared by one-pot method using 1,3,,5-hexatriene conjugated olefin as raw material and reticulated Ti / Pt as anode zero-pole-distance electrolytic cell by changing the ratio of acetonitrile / water.
[0092] A zero-pole-distance electrolytic cell was used, and the acetonitrile / water ratio was adjusted to be 1:3, that is, 125 g of acetonitrile, 375 g of water and 11.25 g of sodium bromide were used as the reaction solution, Nafion 117 was selected as the cation exchange membrane, and other conditions were as in Example 1.
[0093] After 1,2,3,4-diepoxybutane is synthesized by paired electrolysis, the reaction solution undergoes acid dissociation to open the ring, freeze crystallization, and dry to obtain white crystal erythritol. The electrolysis current and current density remain unchanged, and the current The efficiency is 80%, the voltage is 3.8V, the total yield is 76%, and the electrolysis energy consumption is 5921kWh / t.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com