Biomarker for thyroid nodule benign and malignant discrimination, multi-gene joint detection kit and application
A technology of thyroid nodules and biomarkers, which is applied in the field of biomedicine, can solve the problems of complex experimental operation process, low sensitivity, and long detection cycle, and achieve the effect of low template input, stable reaction system, and short detection cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0205] Composition of multi-gene combined detection kit for judging benign and malignant thyroid nodules
[0206] The kit includes detection of target genes (BRAF gene V600E, TERT gene C228T / C250T, KRAS gene G12C / G12V / Q61R, NRAS gene Q61R, HRAS gene Q61R a total of 8 DNA single base mutations, CCDC6-RET, NCOA4-RET, PAX8 -PPARG, ETV6-NTRK3, a total of 4 gene fusions) and internal reference genes ACTB, NONO detection reaction solution, PCRMix, RT mixed enzyme, positive control.
[0207] 1. Primer probe combination
[0208] The detection reaction solution includes a total of 8 DNA single-base mutations, CCDC6-RET, NCOA4- RET, PAX8-PPARG, ETV6-NTRK3 (a total of 4 gene fusions) and primer probe compositions of the internal reference genes ACTB and NONO.
[0209] In this example, BRAF(NG_007873.3), TERT(NG_009265.1), KRAS(NG_007524.2), NRAS(NG_007572.1), HRAS(NG_007666.1) and ACTB(NG_007992.1) gDNA sequences are used as templates respectively , designed the respective specific pr...
Embodiment 2
[0276] Application method of multi-gene combined detection kit for judging benign and malignant thyroid nodules
[0277] The use of the multi-gene combined detection kit for judging benign and malignant thyroid nodules includes the following steps:
[0278] 1. Collect fine needle aspiration samples of thyroid nodules from patients to be tested, extract genomic DNA and total RNA, and store them at -20°C for later use. It is recommended to use the tissue cell DNA / RNA extraction kit (spin column method) (Shanghai Ruijing Biotechnology Co., Ltd., China) to extract nucleic acids according to the instructions.
[0279] 2. Prepare a PCR reaction system (the composition is shown in Table 14) with the DNA, RNA, positive control substance of the kit and blank control substance obtained above as templates respectively, and then carry out PCR reaction after fully mixing.
[0280] Table 14 qPCR reaction system
[0281]
[0282] PCR reaction conditions: the first stage: 50°C for 15min,...
Embodiment 3
[0293] Detection limit test of multi-gene combined detection kit for the discrimination of benign and malignant thyroid nodules
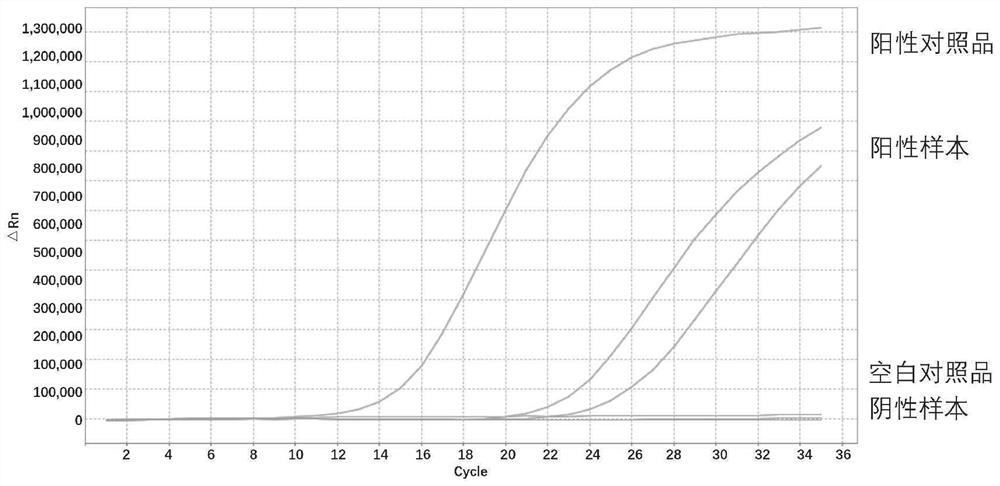
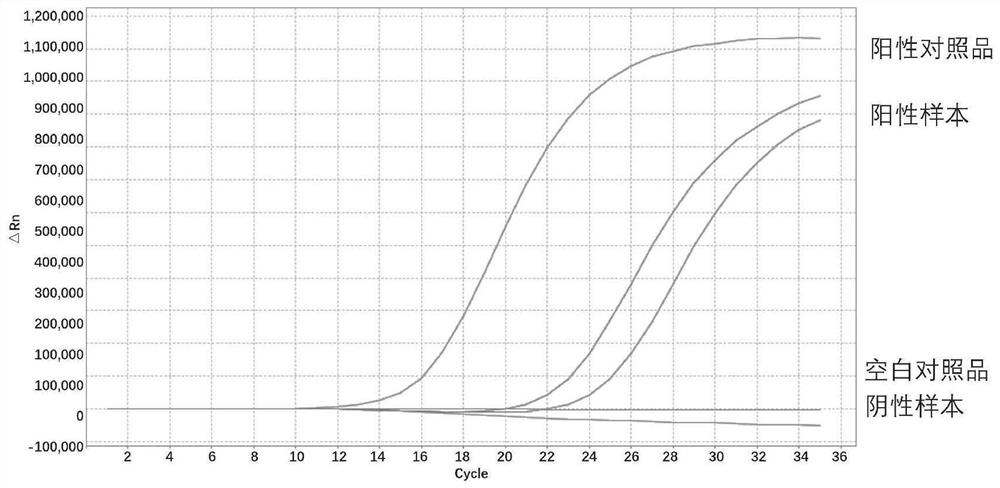
[0294] Select 12 locus positive samples or standards in the kit, of which 8 DNA mutation locus positive samples are diluted to 1% mutation abundance, and the concentrations are: 10ng / μL, 5ng / μL, 2.5ng / μL, 1ng / μL . The four fusion-positive samples were diluted to different copy number concentration gradients, 666 copies / μL, 333 copies / μL, 166 copies / μL, 83 copies / μL (corresponding to the total input amount of 2000 copies, 1000 copies, 500 copies, 250 copies) copy). Use the kit to dilute the template for detection, and repeat each gradient three times to confirm the detection sensitivity of the kit. The detection template gradient and detection results of each site are as follows:
[0295] For BRAF V600E
[0296]
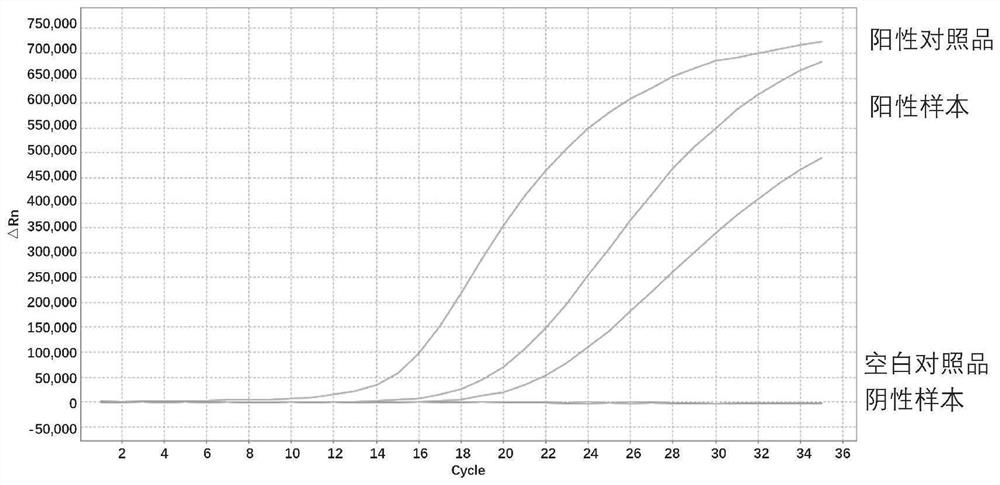
[0297] The detection limit gradient test results of BRAF V600E are as follows Figure 13A shown.
[0298] For TERT C228T:
[0299...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com