Antimicrobial composition
A composition, compound technology, applied in the direction of chemicals for biological control, biocides, animal repellants, etc., can solve the problem of alternatives reducing the risk of microbial resistance development, insensitivity to antimicrobial compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] Measurement of antimicrobial activity of compositions according to the invention
[0171] Methods for Assessing Antimicrobial Activity
[0172] The following methods allow for a combined assessment of the antimicrobial activity of different raw materials. The fractional inhibitory concentration index (FIC index) is a measure of activity (Garcia L.S., Clinical Microbiology Procedures Handbook, pg. 140-162, 3 rd Edition (2010), ASM Press, Washington DC), and calculated according to the following formula:
[0173]
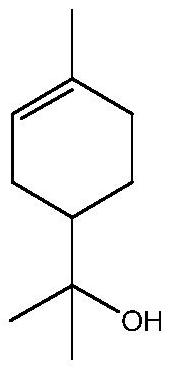
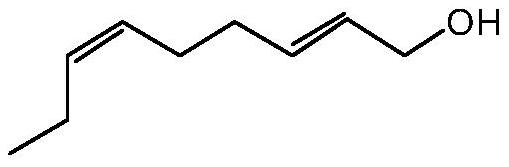
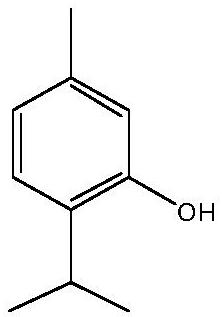
[0174] of which (MIC A individually) and (MIC B alone) are the minimum inhibitory concentrations (MIC) of components A and B when used alone; while (MIC A combination) and (MIC B combination) is the minimum inhibitory concentration of materials A and B when tested in combination. Component A corresponds to non-2,6-dien-1-ol and component B represents 3-neopentylpyridine, 2-methylhex-3-one oxime, terpineol or 2-isopropyl-5- methylphenol.
[0175]...
Embodiment 2
[0221] Antimicrobial Effects of Compositions According to Some Forms Provided herein in Liquid Soap Bases
[0222] Preparation of bacterial solutions
[0223] Bacterial solutions of the two strains E. coli DSMZ 1103 and S. aureus DSMZ 1104 were prepared for BCT testing as follows. Stock cultures stored at -80°C were subcultured on Tryptic Soy Agar (TSA) agar plates and incubated at 37°C for 24 hours to obtain single colonies. Single colonies of primary cultures were streaked onto TSA slants and incubated in a 37°C incubator for 24 hours. Collect bacterial plateaus of slant cultures in PBS buffer to prepare target levels of 1–5 × 10 8 CFU / mL suspension. A 1:100 dilution of each cell suspension in PBS buffer was used as the bacterial solution for the BCT test.
[0224] Preparation of test samples
[0225] Test samples of bases with synergistic binary mixtures were prepared as follows. The raw materials of the synergistic binary mixture (Table 17) were mixed with the ...
Embodiment 3
[0243] Antibacterial effect of compositions according to some forms provided herein in roll-on deodorant bases
[0244] Preparation of bacterial solutions
[0245]A bacterial solution of C. xerosis ATCC 373 strain was prepared for BCT testing as follows. Stock cultures stored at -80°C were subcultured on tryptic soy agar medium containing 0.5% Tween 80 (TSA-TW80) and incubated at 37°C for 48 hours. Primary cultures were subcultured onto TSA-TW80 again to prepare secondary cultures. A single colony of the secondary culture was inoculated into 30 ml of brain heart infusion (BHI) broth (BHI-TW80) containing 0.5% Tween 80 and incubated at 37°C for 24 hours at 180 rpm. An aliquot (1 ml) of the 24 hour culture was inoculated into 30 ml of fresh BHI-TW80 broth and incubated for 48 hours at 37°C at 180 rpm. Aliquots (2-3 ml) of the 48 hour culture were inoculated into four 50 ml fresh BHI-TW80 broth media and incubated at 37°C at 180 rpm for 4-6 hours. When the OD reached the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com