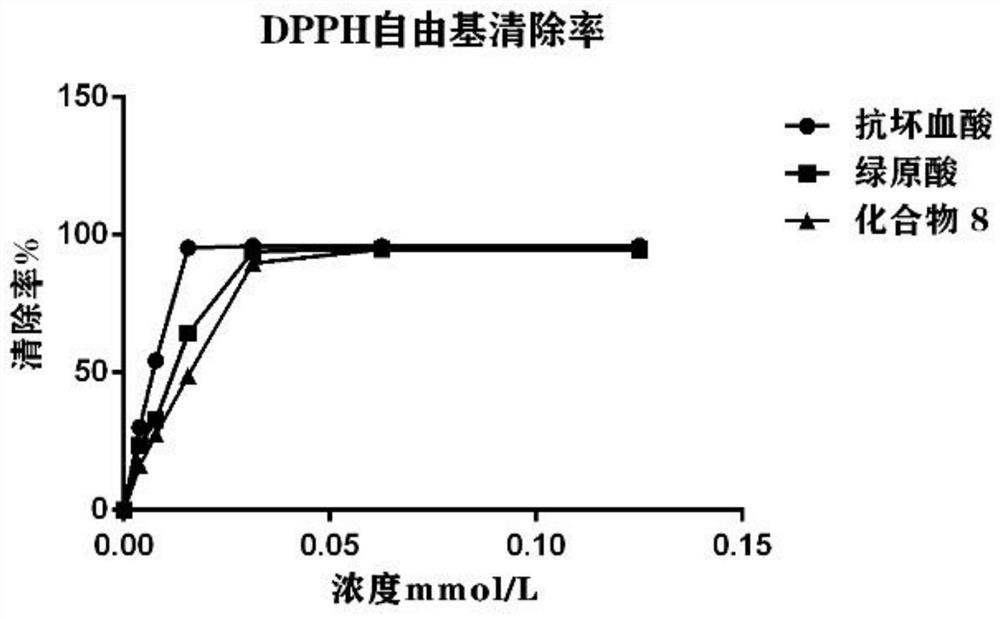
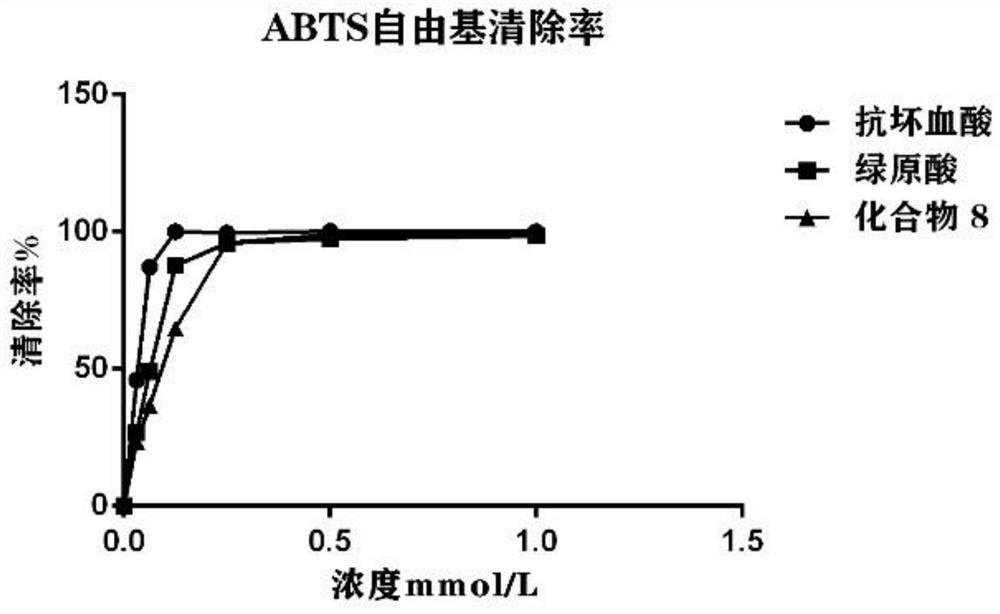
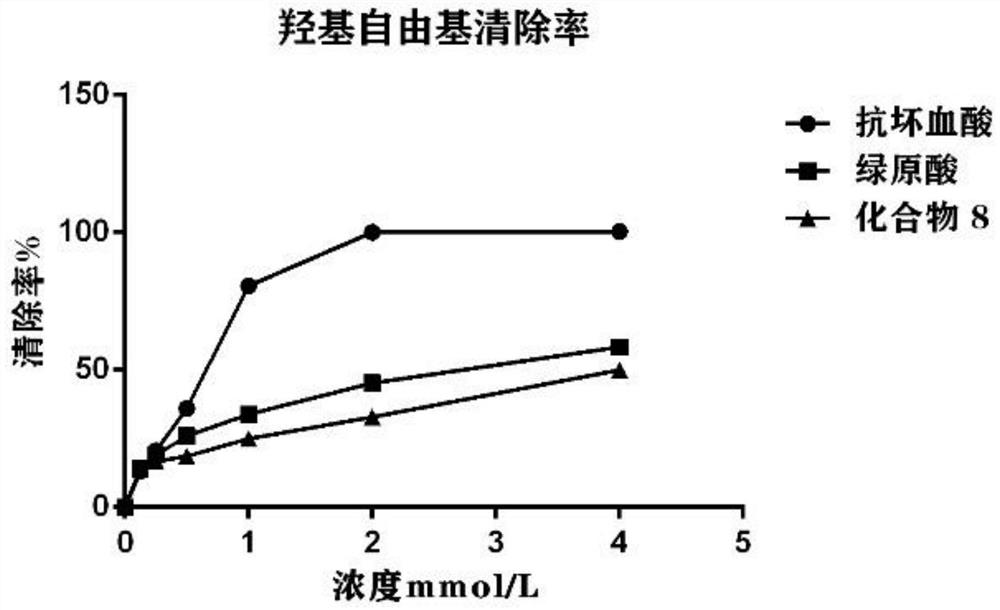
Chlorogenic acid analogue as well as preparation method and application thereof
A technology of analogs and chlorogenic acid, which is applied in the field of chlorogenic acid analogs and its preparation, can solve problems such as easy hydrolysis and instability of chlorogenic acid, achieve strong antioxidant activity, and have a simple preparation method, which is conducive to popularization and use Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The present embodiment provides a chlorogenic acid analog, which is the following compound:
[0072]
[0073] The synthetic route of compound 8 is as follows:
[0074]
[0075] The preparation method of compound 8 includes:
[0076] a) Synthesis of compound 2:
[0077]
[0078] DMAP (0.338 g, 2.77 mmol) was weighed and dissolved in acetic anhydride (26 mL, 277 mmol), so that DMAP and acetic anhydride were fully reacted; then, compound 1 (quinic acid, 5 g, 27.7 mmol) was added, and the reaction system was added DIPEA (48 mL, 277 mmol). TCL monitoring: (DCM:methanol=10:1); After the reaction is over, NaHCO 3 The pH of the aqueous solution was adjusted to alkaline, and EA was used to extract impurities (discarded); 2mol / L HCl was adjusted to pH=2, EA was extracted, and anhydrous Na 2 SO 4 After drying, suction filtration, and spin drying, compound 2 was obtained with a yield of 100%, which was directly used in the next reaction without purification.
[0079] b...
Embodiment 2
[0099] This embodiment provides another chlorogenic acid analog, and the chlorogenic acid analog is the following compound:
[0100]
[0101] The synthetic route of compound 9 is as follows:
[0102]
[0103] Steps a) to e) in the preparation method of compound 9 are the same as steps a) to e) in the preparation method of compound 8, and the only difference is:
[0104] Step f) in the preparation method of compound 9 is as follows:
[0105] f) Synthesis of compound 9:
[0106]
[0107] Compound 7 (2.4 g, 3.5 mmol) was weighed and dissolved in 10 mL of methanol; triethylamine (10 mL, 2 mmol) was weighed and added to the reaction solution. TLC monitoring (DCM:MeOH=10:1), after the completion of the reaction, the crude product of compound 9 was obtained by removing the organic solvent. The crude product was purified by column chromatography (DCM:MeOH=40:1) to obtain pure compound 9 with a yield of 78%.
[0108] Hydrogen NMR spectrum of compound 9: 1 H NMR (400MHz, c...
Embodiment 3
[0110] This embodiment provides another chlorogenic acid analog, and the chlorogenic acid analog is the following compound:
[0111]
[0112] The synthetic route of compound 10 is as follows:
[0113]
[0114] Steps a) to f) in the preparation method of compound 10 are the same as steps a) to f) in the preparation method of compound 9, and the only difference is:
[0115] The preparation method of compound 10 also includes step g) as follows:
[0116] g) Synthesis of compound 10:
[0117]
[0118] Compound 9 (100 mg, 0.165 mmol) was weighed and dissolved in 10 mL of DMF; potassium carbonate (91.2 mg, 0.66 mmol) was weighed and added to the reaction solution, followed by methyl iodide (234 mg, 1.65 mmol), and the reaction was stirred at 80°C. TLC monitoring (DCM:MeOH=30:1), after the completion of the reaction, the reaction solution was diluted with water, extracted with EA 3 times, washed with saturated brine twice, the organic phases were combined and dried over an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com