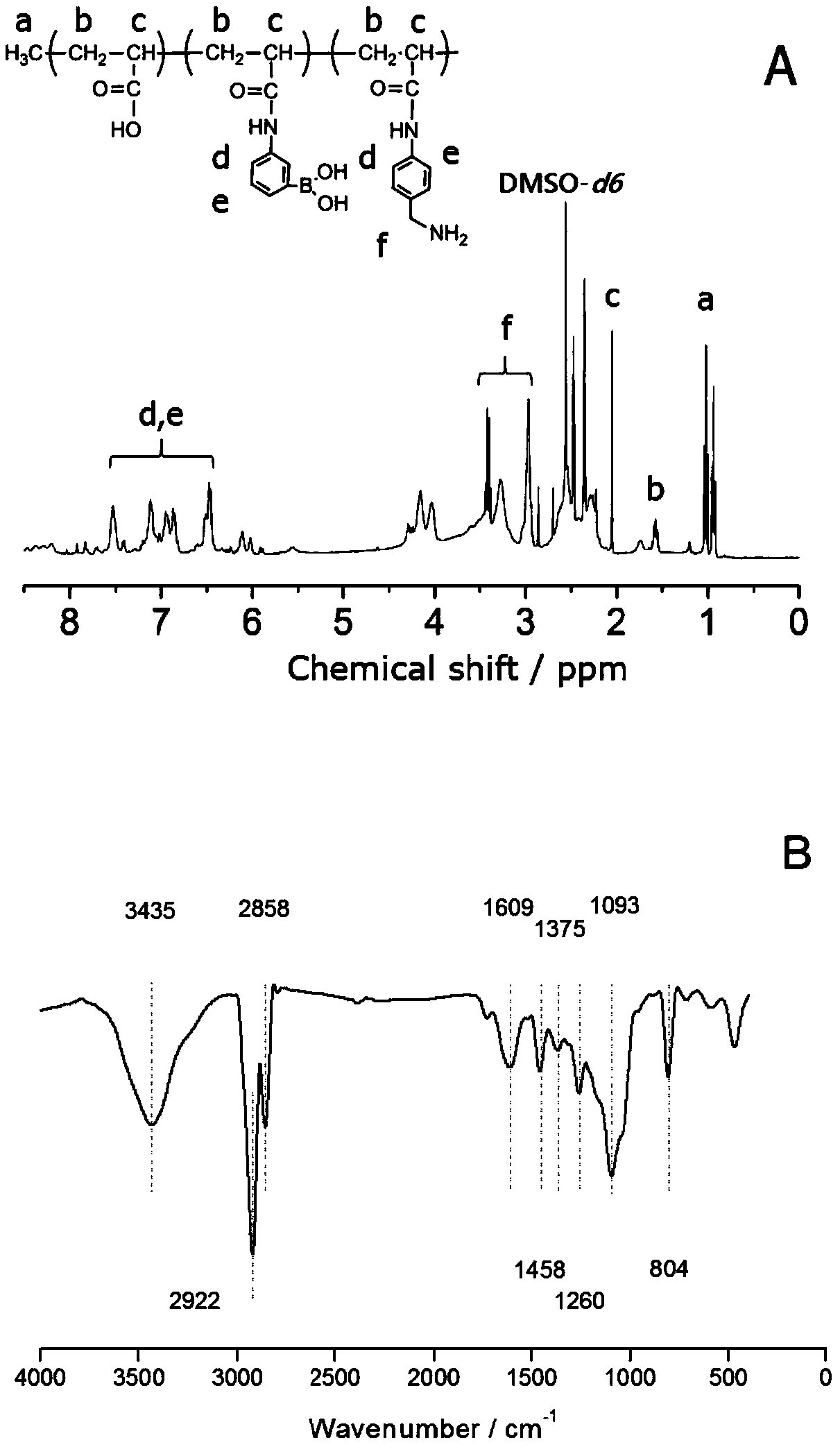
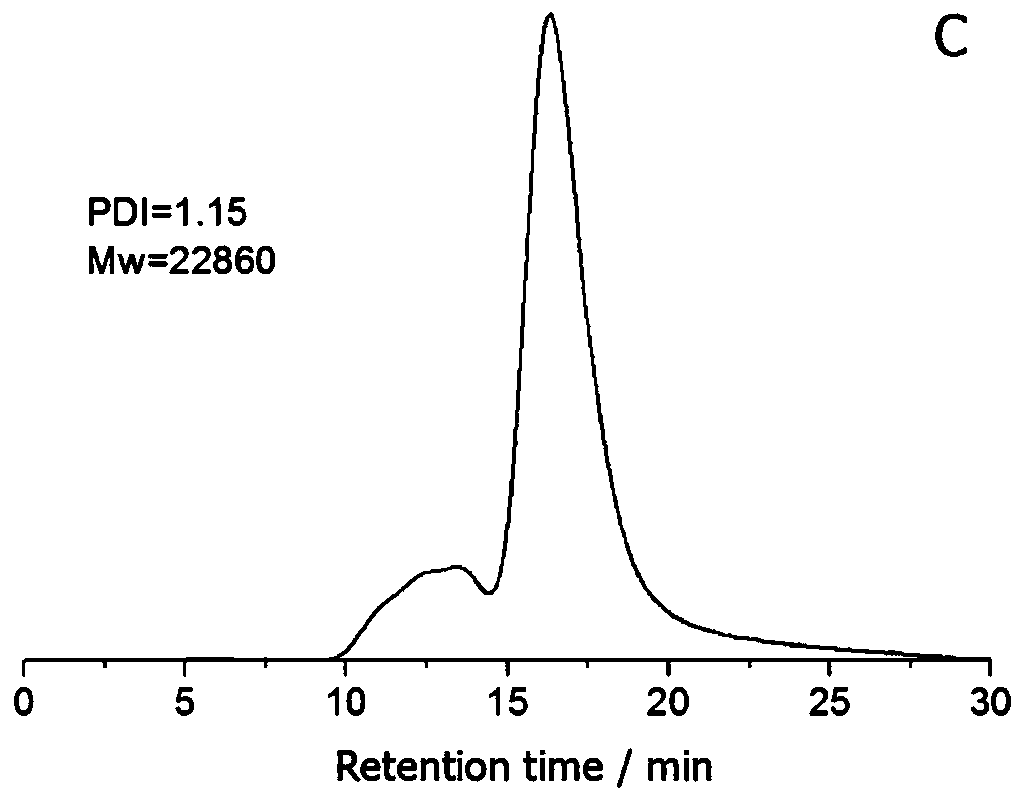
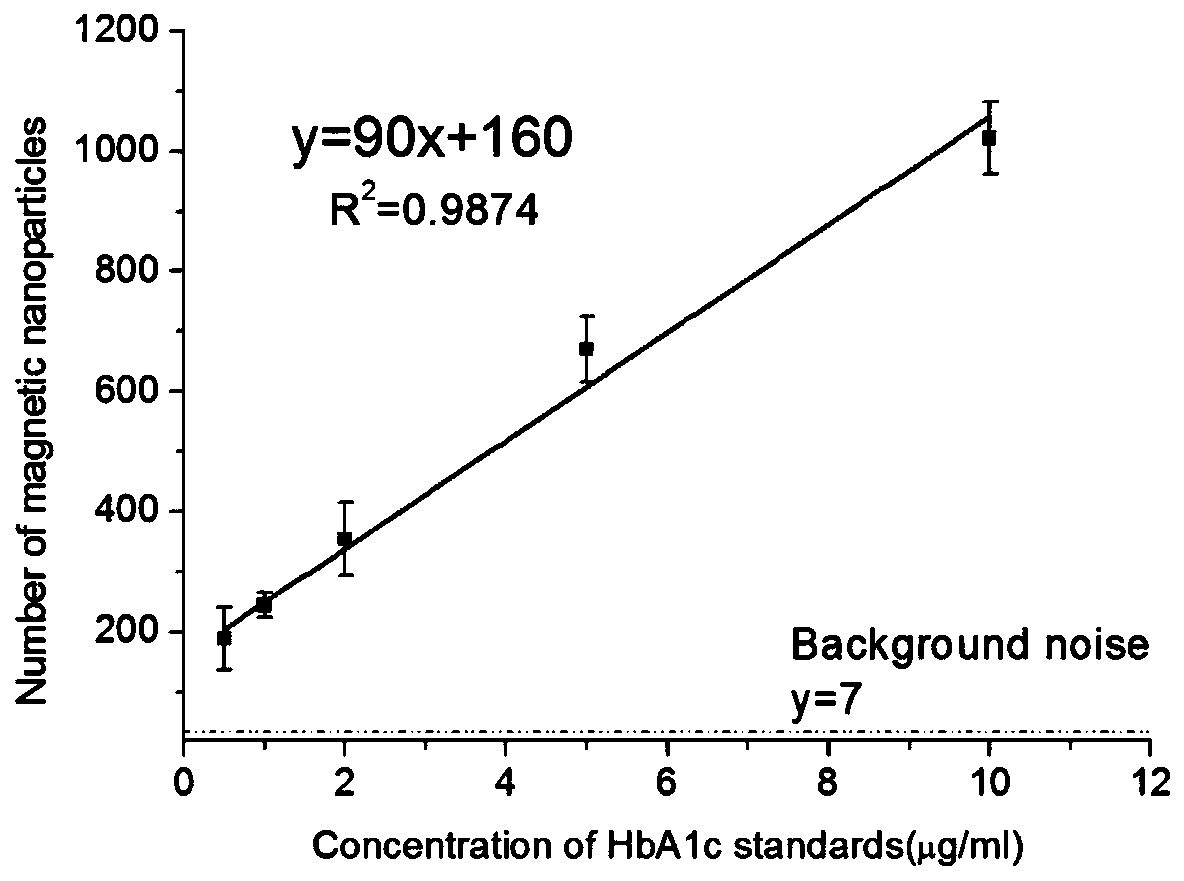
A kind of acrylamidophenylboronic acid polymer and its preparation and application
A technology of acrylamidophenylboronic acid and aminophenylboronic acid, which is applied in the application field of small molecules or glycoproteins, can solve the problems of boronic acid group recognition and enhancement of glycoproteins, and achieves easy separation and purification, moderate degree of polymerization, and simple synthesis steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] In a 50 mL three-necked flask, 4.5 mmol of acrylic acid was dissolved in 15 mL of dimethylformamide, followed by adding 4.5 mmol of N,N'-dicyclohexylcarbodiimide (EDC) and 4.5 mmol of N-hydroxysuccinimide (NHS) in Activated in an ice-water bath under nitrogen protection for 4 h. Weigh 1.5 mmol of 3-aminophenylboronic acid monohydrate, 3 mmol of 4-aminobenzylamine dihydrochloride and 12.5 mmol of sodium hydroxide in 15 mL of dimethylformamide / water (v / v 3:2) mixed solvent , It was quickly injected into a three-necked flask under vigorous stirring, and the reaction was continued for 12h. After the amidation was completed, 2.25 mmol of acrylic acid and 0.15 mmol of azobisisobutyronitrile were added to the solution, and heated to 70° C. for 24 h under nitrogen protection. After the reaction was completed and returned to room temperature, about 30 mL of the solution was slowly added dropwise to 1 L of acetone, stirred rapidly to obtain a precipitate, filtered with suction a...
Embodiment 2
[0050] The polymer synthesized in Example 1 was diluted with CB buffer solution (pH 9.6), and an appropriate amount of moisturizing agent and catalyst were added to prepare a spotting solution. Use a pipette gun to spot a sample at a volume of 0.5 μL per point on a silicon wafer spin-coated with a polystyrene-grafted maleic anhydride film, and place the spotted silicon wafer under the condition of 70% humidity for 20 min. Then move to 10% humidity and dry for 30min. Soak the polymer-coated silicon wafer in CBT solution (pH 9.6) for 5 minutes until all unreacted maleic anhydride is completely hydrolyzed. After taking it out, absorb the surface liquid on one side of the silicon wafer with absorbent paper. Add glycosylated hemoglobin solution (20 μL) with a concentration of 0.5-10 μg / mL to the silicon wafer, rinse the surface antigen solution with eluent after reacting for 5 minutes, and absorb the surface liquid on one side of the silicon wafer with absorbent paper. Add 20 μL o...
Embodiment 3
[0052] In a 50 mL three-necked flask, 9 mmol of acrylic acid was dissolved in 15 mL of dimethylformamide, followed by adding 4.5 mmol of N,N'-dicyclohexylcarbodiimide (EDC) and 4.5 mmol of N-hydroxysuccinimide (NHS) in Activated in an ice-water bath under nitrogen protection for 4 h. Weigh 1.5mmol of 3-aminophenylboronic acid monohydrate, 3mmol of 4-aminobenzylamine dihydrochloride and 12.5mmol of sodium hydroxide in 15ml of dimethylformamide / water (v / v 3:3) mixed solvent , It was quickly injected into a three-necked flask under vigorous stirring, and the reaction was continued for 12h. After the amidation was completed, 0.3 mmol of azobisisobutyronitrile was added to the solution, and heated to 70° C. for 24 h under the protection of nitrogen. After the reaction was completed and returned to room temperature, about 30 mL of the solution was slowly added dropwise to 1 L of acetone, stirred rapidly to obtain a precipitate, filtered with suction and washed several times with et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com