Preparation method of ribociclib intermediate
A technology of Ribociclib and intermediates, applied in the field of drug synthesis, can solve the problems of unsuitability for industrialized large-scale production, lengthy reaction steps, and low total yield, and achieve great implementation value and social and economic benefits, reasonable cost, and low price low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
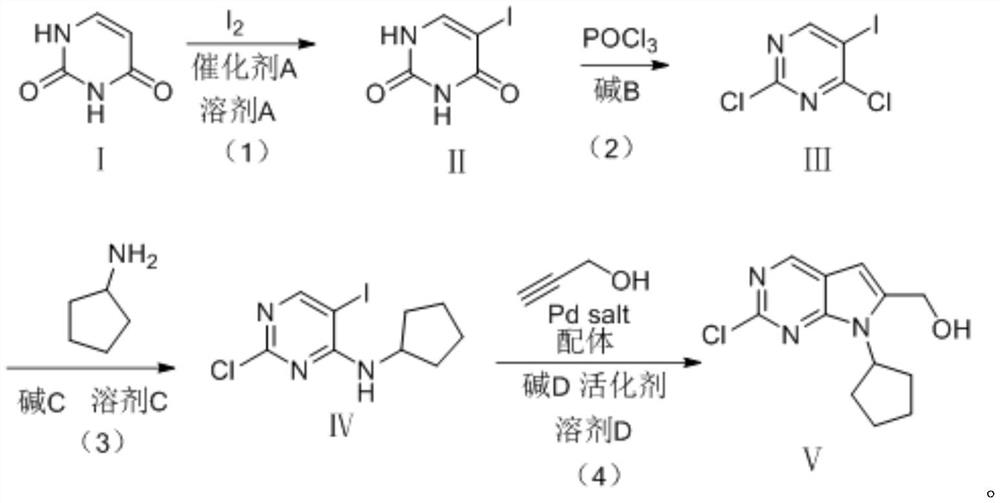
[0047] As a kind of preferred embodiment, a kind of preparation method of ribociclib intermediate, specifically comprises the steps:
[0048] (1) Using uracil as the starting material and iodine as the iodine reagent, compound I, iodine and ceric ammonium nitrate were added to solvent A, and the temperature was raised to 70-80°C, and the reaction was kept for 5-6h to obtain compound II: 5- Iodine-uracil; molar ratio of compound I, iodine and catalyst A=1.0:0.5-1.0:0.05-0.15.
[0049] (2) Add base B to compound II and phosphorus oxychloride, and heat the temperature to 90-100° C. to obtain compound III: 5-iodo-2,4-dichloropyrimidine; the base B is selected from triethylamine, N , one of N-dimethylaniline and N,N-diisopropylethylamine. Compound II, POCl 3 , the substance ratio of alkali B = 1.0: 3.0-10.0: 2.0-5.0.
[0050] (3) Dissolve compound III and base A in solvent A, cool to -10-0°C, add dropwise a mixture of cyclopentylamine and solvent A, and react for 1 to 5 hours to...
Embodiment approach
[0053] A preparation method of ribociclib intermediate, comprising the following steps:
[0054] (1) Compound I, iodine, catalyst A and solvent A are mixed, the temperature is raised to 70-80° C., and the reaction is incubated for 5-6 hours to generate compound II; the catalyst A is selected from the group consisting of DMAP, hydrogen peroxide, and ceric ammonium nitrate; The solvent A is selected from one or more of tetrahydrofuran, water, acetonitrile, ethanol, isopropanol and tert-butanol. The molar ratio of compound I, iodine and catalyst A=1.0:0.5-1.0:0.05-0.15.
[0055] (2) Compound II, phosphorus oxychloride and base B are mixed and reacted to form compound III; the base B is selected from triethylamine, N,N-dimethylaniline and N,N-diisopropylethylamine one or more of them. In step (2), the molar ratio of compound II, phosphorus oxychloride and base B=1.0:3.0-10.0:1.5-5.0.
[0056] (3) Dissolve compound III and base C in solvent C, cool to -10-0°C, add dropwise the m...
Embodiment 1
[0064] 1) Preparation of Intermediate II:
[0065] The ratio of the amount of the feeding material is uracil: iodine: cerium ammonium nitrate = 1: 0.6: 0.1
[0066] In a 1L there-necked flask, add uracil (112g, 1mol), 336mL of acetonitrile, iodine particles (152g, 0.6mol), ceric ammonium nitrate (54.8g, 0.1mol), turn on stirring, be warming up to 80 DEG C of reflux, insulation reaction , HPLC monitoring the consumption of raw materials, the temperature of the reaction solution was naturally cooled to 50 ° C, the acetonitrile was evaporated under reduced pressure, and recovered for use. Add 200 mL of 5% aqueous sodium thiosulfate solution until the solid color fades, filter, rinse the filter cake with water, and collect the filter cake. Product appearance: white solid, mass: 222 g, reaction yield 93%.
[0067] Hydrogen spectrum characterization of intermediate II;
[0068] 1 H NMR (400MHz, DMSO-d 6 , ppm) δ 11.39 (s, 1H), 11.20 (s, 1H), 7.90 (s, 1H).
[0069] 2) Preparati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com